Adolf von Baeyer, a German chemist and a Nobel prize winner from the University of Munich, Germany, in 1885, proposed a theory to explain the relative stability of the first few cycloalkanes. According to this theory, the strain energy in a cycloalkane is directly related to the angle strain and torsional strain present in the ring structure.
The theory is based on the following facts:
- Cyclo-alkanes are saturated compounds. So, all the carbons should have a normal tetrahedral angle of 109.5ᵒ.
- Any deviation of bond angles from the normal tetrahedral value would impose a condition of internal strain on the ring called Angle Strain.
- The higher the value of the angle strain, the more stable the compound is.
- He assumes that all the cyclo-alkanes are flat and coplanar, which means they are two-dimensional and present in one plane.
Baeyer formulated his strain theory based on the notion that stability in cycloalkanes hinges on the degree of strain caused by deviations in bond angles from the ideal tetrahedral angle. Although the theory has undergone extensions and modifications, it retains significance in organic chemistry, offering valuable insights into the stability of carbocyclic compounds.
Cyclopropane:
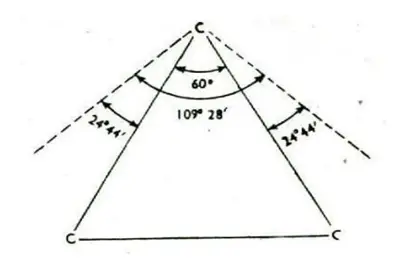
In cyclopropane, the three carbon atoms occupy the corners of an Equilateral Triangle. Thus, cyclopropane has C—C— C bond angles of 60°. This implies that the normal tetrahedral angle of 109.5° between any two bonds is compressed to 60° and that each of the two bonds involved is pulled in by
1/2(109°28’—60°) = 24°44′
The value 24°44′ represents the Angle Strain or the deviation through which each bond bends from the normal tetrahedral direction.
Cyclo-Butane:
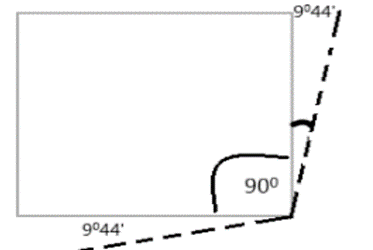
Here, the four carbon atoms occupy the corners of a square. So it has C—C—C bond angles of 90°. The angle Strain on each bond is 1/2(109°28′-90°) = 9°44′
Cyclo-Pentane:
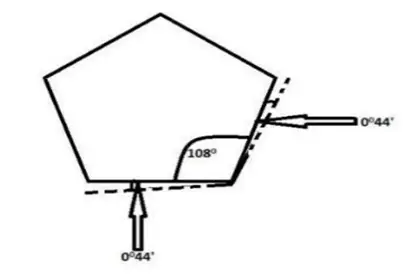
Here, the five carbon atoms lie at the corners of a Regular Pentagon. Thus, cyclopentane has C—C—C bond angles of 108°. The Angle Strain which each bond fills is
1/2(109°28’— 108°) = 0044′
Cyclo-Hexane:
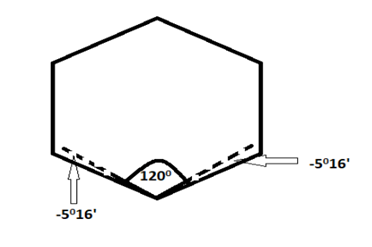
In cyclohexane, the six carbon atoms occupy the corners of a Regular Hexagon. Thus, cyclohexane has
C—C—C bond angles of 120°. The Angle Strain will be
1/2(109°28’— 1200) = -5°16′
Derivation:
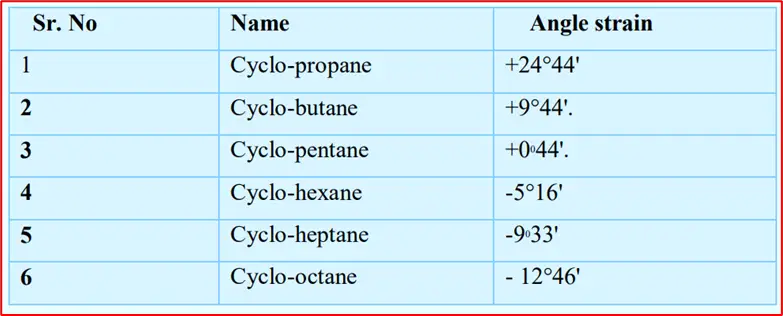
1. The plus sign (+) indicates that the C-C bonds in a ring structure must be compressed to fit the ring’s geometry. Conversely, the minus sign (-) indicates that the C-C bonds must be widened to fit the ring’s geometry. The magnitude of the angle strain, whether positive or negative, determines the degree of strain present in the ring.
2. The maximum deviation from the normal tetrahedral angle occurs in cyclopropane, making it the most unstable. Therefore, it opens up easily, releasing the strain within.
3. The cyclopentane molecule is the least strained and most stable due to its minimal deviation from the normal tetrahedral angle.
4. Baeyer Strain Theory states cyclohexane and higher cycloalkanes are increasingly unstable and more reactive.
Limitation of Baeyer-Strain Theory:
1. The limitation of Baeyer strain theory is that he assumed that all cycloalkanes are planar.
2. Baeyer could not explain the effect of angle strain in the Larger Ring System.
3. According to Baeyer, Cyclopentane should be much more stable than cyclohexane, but practically, it is the reverse. 4. Larger ring systems are not possible, according to Baeyer, as they have negative strain, but they exist and are much more stable. Larger ring systems are not planar but puckered to eliminate angle strain.





One thought on “Baeyer Strain Theory”