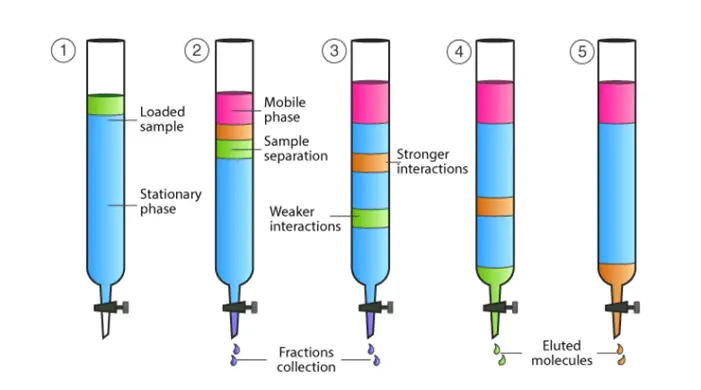
Partition column chromatography is a type of liquid-liquid chromatography where separation is based on the partitioning of components between two immiscible liquid phases—one as the stationary phase and the other as the mobile phase. This technique is commonly used for separating mixtures of compounds with varying solubilities and plays an important role in pharmaceutical, biochemical, and chemical research.
Principle of Partition Column Chromatography
Partition column chromatography is based on the partitioning principle—the separation of components depends on their relative solubility between two immiscible liquids (usually a polar liquid and a non-polar liquid). One of these liquids serves as the stationary phase, coated onto a solid support (like silica or alumina), while the other is the mobile phase.
As the mixture flows through the column, each component distributes itself between the stationary and mobile phases depending on its solubility, leading to separation. Compounds that are more soluble in the mobile phase move faster through the column, while those more soluble in the stationary phase travel more slowly.
Methodology
1. Stationary Phase (Liquid-Coated Solid Support)
–The stationary phase in partition column chromatography is a liquid layer adsorbed onto the surface of an inert solid like silica gel or alumina.
– The stationary phase liquid is often polar (such as water or methanol), which interacts with the sample components based on their polarity.
2. Mobile Phase
– The mobile phase is usually a non-polar or moderately polar solvent (like hexane or ether) that carries the sample through the column.
– The mobile phase is chosen based on the solubility of the compounds being separated. It may be adjusted during the process using either isocratic elution (constant solvent composition) or gradient elution (changing the solvent composition over time).
3. Sample Introduction
– The sample is dissolved in the mobile phase or a small amount of solvent and introduced at the top of the column.
– Proper loading ensures even distribution of the sample in the column, leading to efficient separation.
4. Separation Process
– As the mobile phase flows through the column, the components of the sample interact with both the stationary and mobile phases.
– Compounds that are more soluble in the stationary phase remain retained longer, while those that are more soluble in the mobile phase travel faster.
– The compounds separate based on their partition coefficients between the two phases.
5. Elution and Detection
– The separated components exit the column at different times, called their retention times.
– Fractions of the eluate are collected and analyzed using techniques like spectrophotometry, chromatography detectors (UV, refractive index), or mass spectrometry.
Types of Partition Column Chromatography
1. Liquid-Liquid Partition Chromatography:
– In this type, both the stationary and mobile phases are liquids. The stationary phase is held on a solid support, and separation occurs based on differential partitioning between the two liquids.
2. Gas-Liquid Chromatography (GLC):
– Here, the mobile phase is a gas (like helium or nitrogen), and the stationary phase is a liquid coated on a solid support. This method is used primarily for separating volatile organic compounds.
Factors Affecting Partition Column Chromatography
1. Choice of Stationary and Mobile Phases:
– The polarity and solubility of both phases must be chosen carefully based on the nature of the compounds being separated.
2. Flow Rate:
– A slower flow rate allows better partitioning between the phases, leading to sharper separation, while faster flow rates may cause band broadening.
3. Temperature:
– Temperature can influence the partition coefficient by changing the solubility of the components in the mobile and stationary phases.
4. Column Length and Diameter:
– A longer column provides more surface area for partitioning and leads to better separation, but it also increases the time required for the process.
Advantages of Partition Column Chromatography
1. High Efficiency:
– Offers high-resolution separation of complex mixtures.
2. Flexibility:
– The choice of solvents and operating conditions can be easily modified to suit different compounds.
3. Gentle Separation:
– Ideal for separating heat-sensitive compounds such as proteins and nucleic acids, since it can be performed at room temperature.
4. Scalability:
– Can be used for both analytical and preparative-scale separations, making it applicable in research and industrial settings.
Disadvantages of Partition Column Chromatography
1. Slow Process:
– The separation can take a long time, especially for compounds with very similar solubilities.
2. Maintenance of Stationary Phase:
– The stationary phase can degrade over time, especially if the liquid layer is not replenished, leading to reduced efficiency.
3. Limited to Soluble Compounds:
– Partition chromatography is not suitable for separating compounds that are not soluble in the chosen solvent system.
4. Contamination and Carryover:
– There is a risk of contamination or carryover of compounds between runs if the column is not properly cleaned.
Applications of Partition Column Chromatography
1. Pharmaceutical Industry:
– Widely used for separating and purifying drug components, especially when precise separation of active ingredients from impurities is required.
2. Biochemistry:
– Used for the separation of amino acids, proteins, and nucleotides, as well as for the purification of biomolecules.
3. Natural Product Isolation:
– Effective in separating plant extracts, essential oils, and other naturally occurring compounds based on their solubility properties.
4. Environmental Analysis:
– Applied in the detection and separation of pollutants, pesticides, and other contaminants in environmental samples.
5. Food Chemistry:
– Used for the analysis of food additives, vitamins, and preservatives to ensure product safety and quality.