1. Introduction to Bromometry
Bromometry is a type of redox volumetric analysis that involves the use of bromine (Br₂) or bromine-containing compounds as titrants to determine the concentration of reducing agents in a solution. The titration involves the generation of bromine from an oxidizing agent, which is then titrated with a reducing agent, such as sodium thiosulfate, until the endpoint is reached.
Bromometry is particularly useful for determining substances that can act as reducing agents, including halides like iodide (I⁻) and certain organic compounds, by using bromine as the oxidant.
2. Principle of Bromometry
The principle behind bromometry is based on the oxidation-reduction reaction between bromine (Br₂) and a reducing agent. In the presence of a reducing agent, bromine is reduced to bromide ions (Br⁻), and the reducing agent is oxidized. The bromine (Br₂) is usually generated in situ from a bromine source (such as bromine water or potassium bromate) and reacts with the sample to form bromide ions (Br⁻).
Generation of Bromine:–
Bromine is commonly generated from potassium bromate (KBrO₃) in acidic conditions. In the presence of an acid and a reducing agent, bromine (Br₂) is formed from the bromate.

Reduction of Bromine:
The bromine (Br₂) is then titrated with a reducing agent, such as sodium thiosulfate (Na₂S₂O₃), to reduce the bromine to bromide (Br⁻).
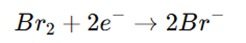
The endpoint of the titration is typically detected using an indicator such as starch, which forms a blue complex with iodine produced in the titration reaction.
3. Procedure of Bromometry
The typical steps for performing a bromometric titration are as follows:
(a) Preparation of the sample:
Prepare the sample solution containing the reducing agent to be determined. The sample is typically dissolved in an acidic medium (often sulfuric acid) to facilitate the reaction with bromine.
(b) Generation of bromine:
- Bromine is usually generated in situ from potassium bromate (KBrO₃) or from bromine water in the presence of an acid.
- The reaction to generate bromine is:

(c) Addition of excess bromine:
- Add an excess of the generated bromine to the sample solution. The bromine reacts with the reducing agent in the sample, forming bromide ions (Br⁻).
- This step is carried out in a controlled manner to ensure that the amount of bromine added is enough to react with all the reducing agents.
(d) Titration with sodium thiosulfate:
- Fill a burette with a standard solution of sodium thiosulfate (Na₂S₂O₃).
- Titrate the excess bromine with sodium thiosulfate. The sodium thiosulfate reduces the remaining bromine to bromide ions (Br⁻).
- The endpoint is approached when the yellow color from bromine fades, and the solution becomes colorless.
(e) Use of starch indicator (optional):
To detect the endpoint more clearly, a starch indicator can be added toward the end of the titration. If iodine is present, it forms a blue complex with starch. The blue color disappears when all the iodine is reduced to iodide, indicating that the titration is complete.
(f) Calculation:
- The amount of sodium thiosulfate used is related to the concentration of bromine and, by extension, to the concentration of the reducing agent in the sample.
- The molarity of the reducing agent is determined based on the volume of sodium thiosulfate used and its molarity:
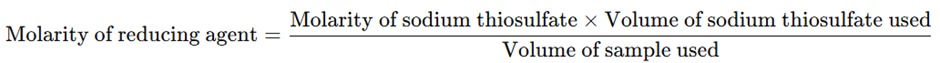
4. Applications of Bromometry
Bromometry is used in a variety of chemical analyses to determine the concentration of reducing agents. Some key applications include:
(a) Determination of Iodide (I⁻) Ions:
Bromometry can be used to determine the concentration of iodide (I⁻) in a sample. In this case, the bromine (Br₂) reacts with iodide ions to form iodine (I₂), which is then titrated with sodium thiosulfate.
(b) Analysis of Hydrogen Peroxide (H₂O₂):
Bromometry is commonly used to determine the concentration of hydrogen peroxide (H₂O₂), which acts as a reducing agent. Bromine generated from potassium bromate or bromine water reacts with hydrogen peroxide to form bromide ions.
(c) Analysis of Oxidizing Agents in Water:
Bromometry is used for the determination of oxidizing agents, such as chlorine or ozone, in water samples. These oxidizing agents react with the bromine, and the excess bromine is then titrated with sodium thiosulfate.
(d) Determination of Reducing Sugars:
Certain reducing sugars (e.g., glucose, fructose) can be analyzed using bromometry, as they act as reducing agents and can react with bromine in the presence of acid.
(e) Analysis of Organic Compounds:
Bromometry can be used in the determination of certain organic compounds that act as reducing agents, such as some antioxidants in pharmaceuticals and food products.
5. Advantages and Limitations
Advantages:
- Effective for specific reducing agents: Bromometry is a highly effective method for determining reducing agents that readily react with bromine.
- Simple and cost-effective: The method is relatively simple and does not require expensive equipment.
- Widely applicable: Bromometry can be applied to a variety of substances, including inorganic and organic reducing agents.
Limitations:
- Interference from other substances: Other reducing agents or oxidizing agents present in the sample can interfere with the titration, affecting the accuracy of the results.
- Bromine handling: Bromine is a hazardous and volatile substance, requiring careful handling and storage.
- Requires careful endpoint detection: The endpoint can be difficult to detect without the proper use of indicators, particularly if the reducing agent is present in low concentrations.
In summary, bromometry is a valuable analytical technique for determining the concentration of reducing agents in various samples. By generating bromine in situ and titrating it with sodium thiosulfate, bromometry offers a precise and effective method for chemical analysis, with applications ranging from environmental monitoring to pharmaceutical quality control.