Meta Description
Discover the preparation, properties, and medicinal uses of copper sulphate. Learn its synthesis, assay methods, and benefits. Read now for full details!
Introduction
Copper sulphate (CuSO₄) is an essential inorganic compound widely used in medicine, agriculture, and industrial applications. It is primarily available as copper sulphate pentahydrate (CuSO₄·5H₂O), a blue crystalline solid. In this guide, we will explore how copper sulfate is prepared, its key properties, assay methods, and medicinal benefits.
General Methods of Preparation of Copper Sulphate
Copper sulphate can be prepared using several industrial and laboratory methods:
1. Direct Reaction Method: Copper metal reacts with sulfuric acid (H₂SO₄) in the presence of oxygen.
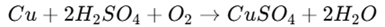
This method is widely used for industrial production.
2. Reaction of Copper Oxide with Sulfuric Acid: Copper(II) oxide (CuO) reacts with dilute sulfuric acid, forming copper sulphate. Commonly used in laboratories.
3. Leaching of Copper Ore: Copper sulphate is extracted from copper ores (e.g., chalcopyrite) using sulfuric acid. The copper ions dissolve and later crystallize as CuSO₄·5H₂O.
Assay of Copper Sulphate
The assay of copper sulphate (CuSO₄) ensures its purity, quality, and potency in various applications. The most commonly used methods include:
1. Iodometric Titration Method
Principle: Copper sulphate is reduced using potassium iodide (KI), which liberates iodine (I₂). The released iodine is then titrated with sodium thiosulphate (Na₂S₂O₃) until the endpoint is reached.
Reactions:
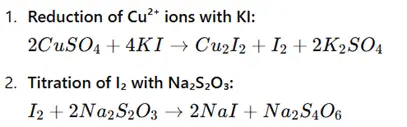
Procedure:
- 1. Dissolve a known amount of copper sulphate sample in water.
- 2. Add excess potassium iodide (KI) solution to liberate iodine.
- 3. Titrate the liberated iodine using standard sodium thiosulphate (Na₂S₂O₃) solution until the brown iodine color fades.
- 4. Use a starch indicator towards the end, where the blue-black color disappears, indicating the endpoint.
- 5. Calculate the purity based on the titration readings.
Endpoint:
- The solution changes from blue to colorless (after adding starch, the blue-black color disappears).
2. Gravimetric Analysis
Principle: Copper is precipitated as cuprous thiocyanate (CuSCN) and weighed to determine purity.
Procedure:
- 1. Dissolve the copper sulphate sample in water.
- 2. Add hydrogen sulfide (H₂S) gas to precipitate copper(II) sulfide (CuS).
- 3. Convert CuS to cuprous thiocyanate (CuSCN) using an appropriate reagent.
- 4. Filter, dry, and weigh the CuSCN precipitate.
- 5. Calculate the copper content based on the weight of CuSCN obtained.
Importance of Assay in Copper Sulphate
- 1. Ensures purity for pharmaceutical, industrial, and agricultural applications.
- 2. Quality control in formulations and chemical manufacturing.
- 3. Regulatory compliance for safety and effectiveness.
Properties of Copper Sulphate
Physical Properties:
Molecular Formula: CuSO₄
Molecular Weight: 159.6 g/mol (anhydrous), 249.7 g/mol (pentahydrate)
Appearance: Blue crystalline solid (pentahydrate), white powder (anhydrous)
Solubility: Highly soluble in water
Melting Point: 110°C (loses water of crystallization)
Chemical Properties:
1. Hydration & Dehydration: Pentahydrate (CuSO₄·5H₂O) loses water at 110°C, forming white anhydrous CuSO₄.
2. Reaction with Alkali: Reacts with sodium hydroxide (NaOH) to form blue copper hydroxide (Cu(OH)₂).
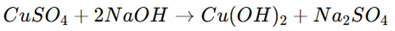
3. Precipitation of Copper: With hydrogen sulfide (H₂S) in an acidic medium, it forms black copper(II) sulfide (CuS).
Medicinal Uses of Copper Sulphate
Copper sulphate has several pharmaceutical and therapeutic applications, including:
1. Emetic Agent: Historically used to induce vomiting in poisoning cases. No longer recommended due to toxicity concerns.
2. Antiseptic and Fungicide: Used as a topical antiseptic to prevent fungal and bacterial infections. Common in footbaths for livestock to prevent hoof rot.
3. Treatment of Copper Deficiency: Used in trace amounts to treat copper deficiency in humans and animals.
4. Astringent in Eye Drops: Historically used in eye drops for its astringent properties.
5. Burn and Wound Healing: Occasionally used in dermatology for wound care due to its antibacterial properties.
Conclusion
Copper sulphate remains a vital compound in medicine, industry, and agriculture. While its medical use has declined due to toxicity concerns, its role as a fungicide, antiseptic, and assay reagent is still significant. Have questions about copper sulphate’s applications?
Drop a comment below or explore our related guides!
Frequently Asked Questions (FAQs)
1. What is copper sulphate used for in medicine?
Answer: It has been used as an emetic, antiseptic, and astringent, but modern use is limited due to toxicity concerns.
2. How is copper sulphate prepared?
Answer: It is prepared by reacting copper metal, copper oxide, or copper ores with sulfuric acid.
3. Is copper sulphate safe for humans?
Answer: In small doses, it is safe for industrial use, but ingestion in large amounts can be toxic.
4. Why is copper sulphate blue?
Answer: The blue color is due to the water of crystallization (CuSO₄·5H₂O).
5. Can copper sulphate be used as an antifungal agent?
Answer: Yes, it is commonly used in fungicide formulations for agriculture and medical applications.



