In the case of benzene, Kekule’s structures (1) and (2) represent the resonance structures. The actual structure of the molecule may be represented as a hybrid of these two resonance structures or by the single structural formula (3).
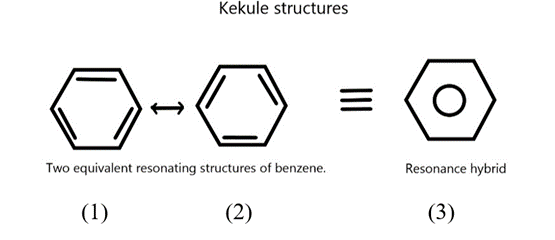
It should be clearly understood that the resonance structures (1) and (2) are not the actual structures of the benzene molecule. They exist only in theory. None of these structures adequately represents the molecule. In resonance theory, we view the benzene molecule as a hybrid of these two hypothetical resonance structures.
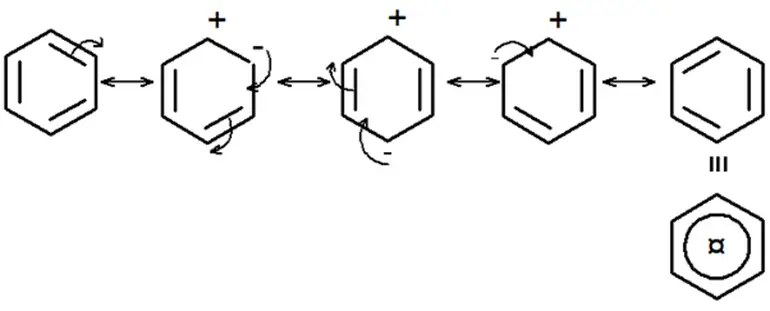
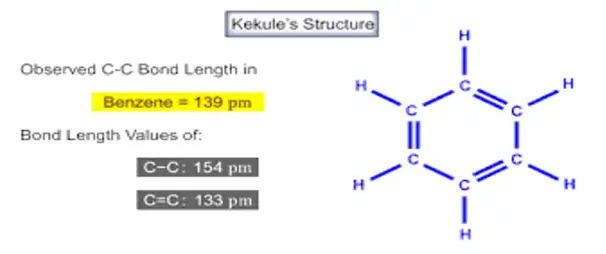
Look at the structures (1 and 2) carefully. All single bonds in structure (1) are double bonds in structure (2), considered a hybrid. Then, benzene’s C-C bonds are neither single nor double bonds. Rather, they are something halfway between. When experimentally exactly found. Spectroscopic measurements show benzene is planar, and all its C-C bonds are of equal length, 1.40Aᵒ. This value lies between the C-C single bond length (1.54Aᵒ) and the C-C double bond length (1.34Aᵒ).
Resonance hybrid is more stable than any of its contributing structures. For benzene, the stability due to resonance is so great that π- bonds of the molecule will normally resist breaking. This explains the lack of reactivity of benzene towards addition.