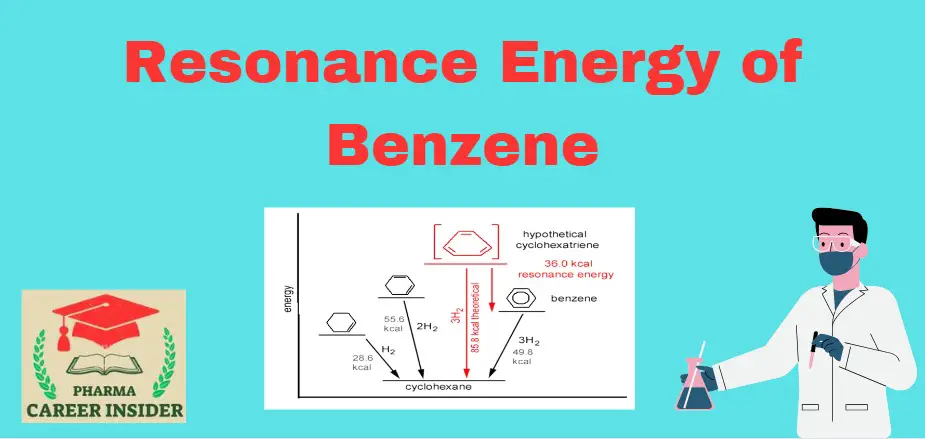
Benzene’s special stability is due to the delocalized π molecular orbital formation. The magnitude of this extra stability can be estimated by measuring the changes in the heat of hydrogenations associated with reactions. Hydrogenation of cyclohexane evolves 28.6 kcal/mol, a value typical for hydrogenation of alkenes.
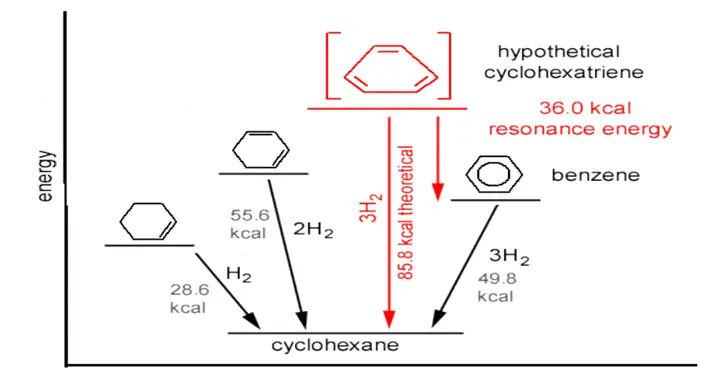
Fig makes these energy relationships more evident. The 36kcal difference between the heat evolved in the hydrogenation of benzene and that estimated for hydrogenation of a compound with three ordinary double bonds is added stability. This added stability is sometimes called Resonance Energy. Resonance energy measures how much more stable a resonance hybrid structure is than its extreme resonance structures.
The resonance energy of benzene is the energy difference between the actual stability of benzene and the hypothetical stability if it had only single and double bonds alternately. This extra stability due to resonance is often expressed in resonance energy.
For benzene, the resonance energy is typically around 36 kcal/mol (kilocalories per mole). This value represents the additional stability gained by the delocalization of electrons over the entire ring, leading to a more uniform electron distribution.
Conclusion
In summary, the resonance energy of benzene reflects the extra stability conferred by the resonance hybrid structure, which allows for the delocalization of electrons and a more uniform electron density throughout the benzene ring.