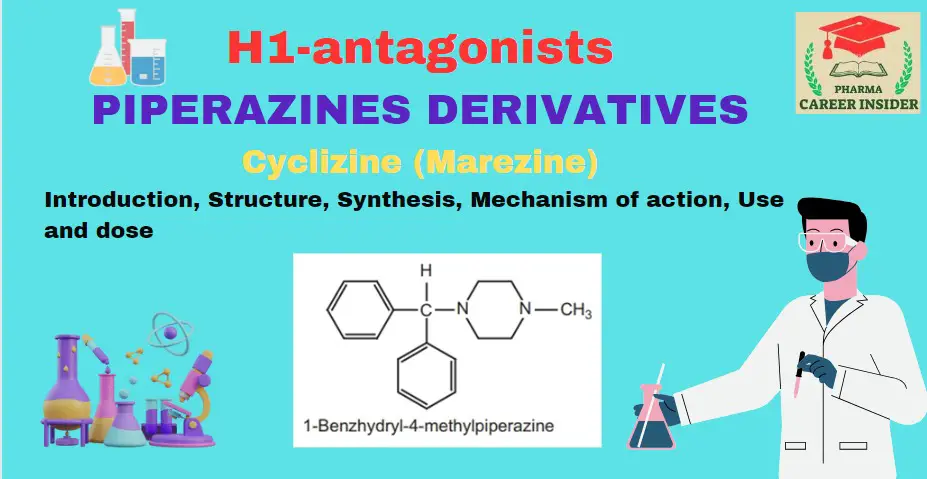
The molar mass of benzene (C6H6) can be calculated by adding the atomic masses of its constituent atoms.
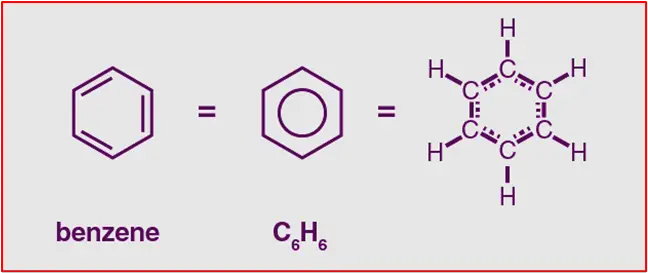
Carbon (C) has an atomic mass of approximately 12.01 g/mol, and hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
So, the molar mass of benzene is:
(6 × atomic mass of carbon) + (6 × atomic mass of hydrogen)
= (6 × 12.01 g/mol) + (6 × 1.01 g/mol)
= 72.06 g/mol + 6.06 g/mol
= 78.12 g/mol
Therefore, the molar mass of benzene is approximately 78.12 grams per mole.