Introduction
An acid-base titration is a quantitative analytical technique used to determine the concentration of an unknown acid or base by reacting it with a standard solution of known concentration. The process is based on the neutralization reaction between an acid and a base, which produces salt and water. The endpoint is usually detected using a pH indicator or a pH meter.
Basic Principle of Acid-Base Titration
After the endpoint is reached, the moles of acid are equal to the moles of base in the reaction mixture. The equivalence point is the theoretical point at which the acid and base are present in stoichiometrically equivalent amounts. The volume of the titrant solution added at the equivalence point can be used to calculate the concentration of the analyte. Titration curves can provide valuable information about the nature of the acid and base being titrated.
The principle of acid-base titration is based on the neutralization reaction:
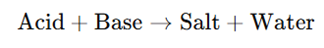
- A strong acid completely ionizes in solution (e.g., HCl → H⁺ + Cl⁻).
- A strong base completely dissociates into its ions (e.g., NaOH → Na⁺ + OH⁻).
- The reaction continues until an equivalence point is reached, where moles of H⁺ = moles of OH⁻.
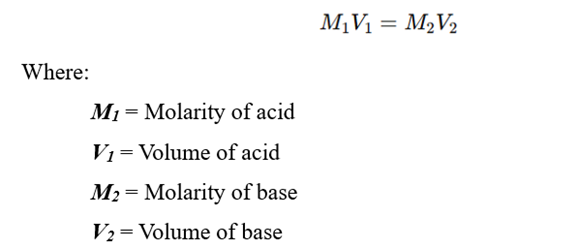
The concentration of the unknown solution can be calculated using the formula:
Types of Acid-Base Titration
Acid-base titrations are classified based on the strength of the acid and base involved:
1. Strong Acid vs. Strong Base
- Example: HCl (Hydrochloric acid) vs. NaOH (Sodium hydroxide)
- pH at equivalence point: 7
- Indicator: Phenolphthalein or Methyl orange
- Sharp endpoint due to complete neutralization.
2. Strong Acid vs. Weak Base
- Example: HCl (Strong acid) vs. NH₃ (Ammonia, weak base)
- pH at equivalence point: Less than 7 (acidic)
- Indicator: Methyl orange
- The weak base does not completely neutralize, leaving the solution slightly acidic.
3. Weak Acid vs. Strong Base
- Example: CH₃COOH (Acetic acid, weak acid) vs. NaOH (Strong base)
- pH at equivalence point: Greater than 7 (basic)
- Indicator: Phenolphthalein
- The weak acid does not completely react, making the final solution slightly basic.
4. Weak Acid vs. Weak Base
- Example: CH₃COOH (Weak acid) vs. NH₃ (Weak base)
- pH at equivalence point: Varies, usually neutral or slightly acidic/basic
- Indicator: No sharp endpoint; pH meter is preferred
- Difficult to perform due to gradual pH changes.
Indicators Used in Acid-Base Titrations
Indicators are chosen based on the expected pH at the equivalence point:
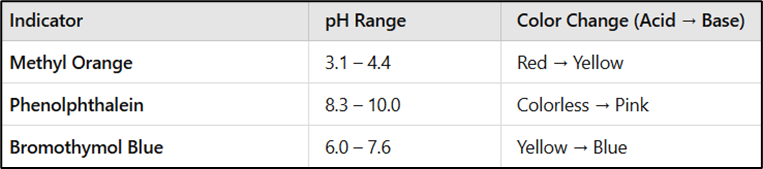
For more accurate results, a pH meter can be used instead of indicators.
Procedure for Acid-Base Titration
1. Preparation of Solutions
- Prepare a standard solution of known concentration (e.g., NaOH for titrating acids).
- Pipette a known volume of the unknown solution into a conical flask.
2. Addition of Indicator
- Add 2-3 drops of a suitable pH indicator to the acid/base solution in the conical flask.
3. Titration Process
- Fill a burette with the standard solution (e.g., NaOH).
- Slowly add the titrant dropwise while swirling the flask.
- Observe the color change of the indicator.
4. Endpoint Determination
- The endpoint is the point where the indicator changes color, signaling neutralization.
- Record the burette reading to determine the volume of titrant used.
5. Calculation of Unknown Concentration
Use the formula:
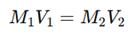
Substituting the values, we can determine the unknown molarity of the acid or base.
Sources of Error and Precautions
- Burette reading accuracy: Always read at eye level to avoid parallax errors.
- Proper mixing: Swirl the flask while adding titrant to ensure a complete reaction.
- Choice of Indicator: Use an appropriate indicator based on the type of acid and base.
- Avoid Over-Titration: Add titrant dropwise near the endpoint to prevent overshooting.
Applications of Acid-Base Titration
- Pharmaceutical Analysis: Used to determine the purity and concentration of drugs.
- Food Industry: Measures the acidity or alkalinity of food products like vinegar and fruit juices.
- Water Quality Testing: Determines the pH and alkalinity of drinking water and industrial effluents.
- Chemical Industry: Used in the manufacturing and quality control of acids, bases, and buffers.
Conclusion
Chemists use acid-base titration as a highly precise method in chemical analysis, pharmaceuticals, environmental testing, and food quality control. Proper techniques, indicators, and calculations ensure accurate and reliable results.