Aromatic carboxylic acids, such as benzoic acid, are stronger acids compared to aliphatic carboxylic acids like acetic acid. This is because benzoic acid has a pKa value of 4.2, which is slightly lower than that of acetic acid (pKa= 4.8).
The carboxylic group in benzoic acid is attached to an sp2 hybridized carbon atom, which is more electronegative than the sp3 hybridized carbon atom in acetic acid.
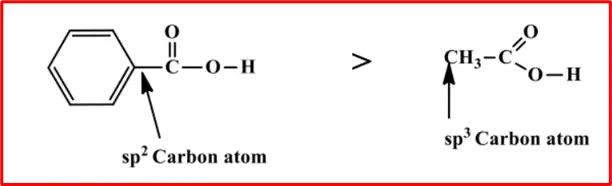
The greater electronegativity of the sp2 hybridized carbon atom is used to explain the resultant or apparent electron-withdrawing inductive effect that the phenyl group demonstrates in stabilizing the carboxylate anion.
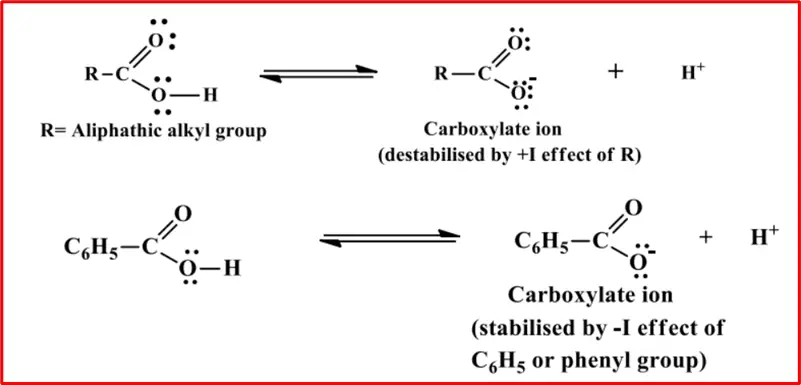
The acidity of substituted benzoic acids depends on the nature and position of the substituents. All the fluoro benzoic acids are more acidic than benzoic acid.
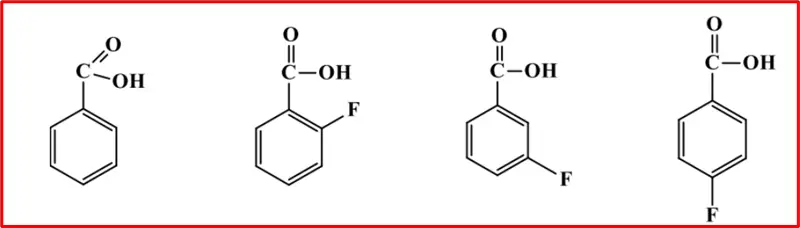
o-fluorobenzoic acid, in which the F-atom is nearest to the carboxylic acid, is the strongest acid of the four; p-fluorobenzoic acid, in which the F-atom is farthest from carboxylic acid, is only slightly more acidic than benzoic acid. The electronegative F-atom exerts/maintains its effect through the bonds and space, withdrawing e– density from the viscinity of the carboxylic group and, hence, stabilizing the carboxylate anion.
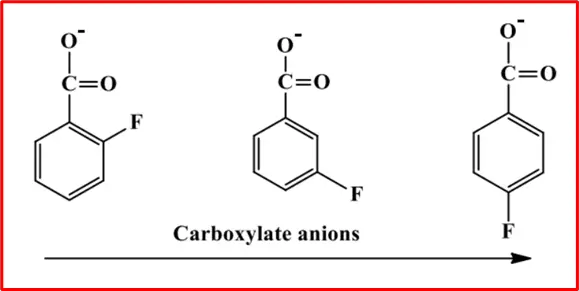
Effect of Substituents on Acidity:
1. Electron-Withdrawing Groups (EWG): Substituents that withdraw electron density from the aromatic ring increase the acidity of aromatic acids. This is because they stabilize the conjugate base formed upon deprotonation. Common electron-withdrawing groups include nitro (-NO2), cyano (-CN), and halogens (-F, -Cl, -Br).
2. Electron-Donating Groups (EDG): Conversely, substituents that donate electron density to the aromatic ring decrease the acidity of aromatic acids. This is because they destabilize the conjugate base. Common electron-donating groups include alkyl groups (-CH3, -C2H5) and methoxy (-OCH3).



