Adsorption chromatography is a separation technique based on the differential adsorption of compounds onto a solid stationary phase. It is widely used to purify and separate chemical compounds based on their interaction with the adsorbent material. The process relies on the principle that different molecules have varying degrees of affinity to the surface of a solid phase (adsorbent) when exposed to a mobile phase (typically a liquid or gas).
Principle of Adsorption Chromatography
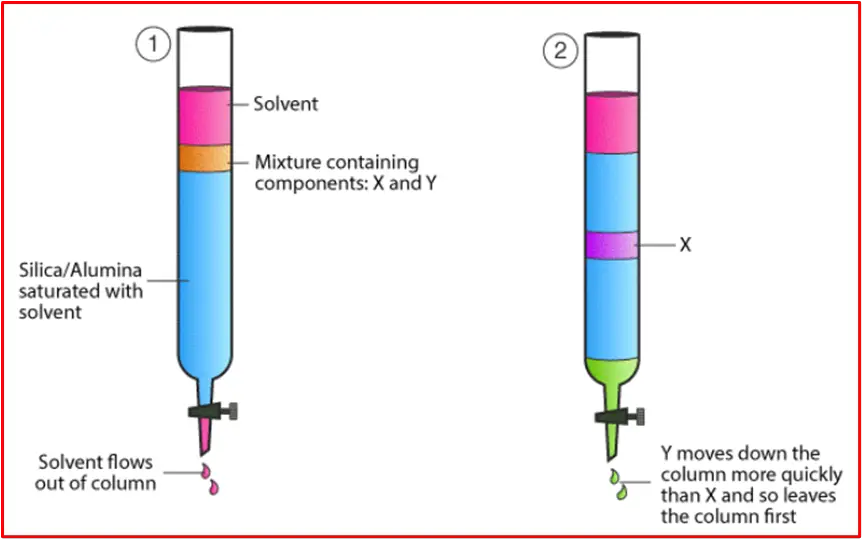
In adsorption chromatography, the separation of components in a mixture occurs due to differences in their adsorption affinity to the stationary phase. The stronger a compound adheres to the surface of the stationary phase, the slower it moves through the column, while compounds with weaker interactions move faster. The differences in retention times lead to the separation of the components.
Methodology
1. Selection of Adsorbent (Stationary Phase):
– Common adsorbents: Silica gel and alumina are the most widely used.
– The adsorbent is packed into a column, where it serves as the stationary phase.
– Particle size and porosity of the adsorbent influence the efficiency of separation. Smaller particles provide better resolution but may require higher pressure to push the mobile phase through.
2. Mobile Phase:
– The mobile phase is a solvent or mixture of solvents that helps to carry the compounds through the column.
– The choice of solvent affects the separation process. A polar solvent increases the elution rate of polar compounds, while a non-polar solvent favors the separation of non-polar compounds.
– Elution techniques: The mobile phase can be varied (gradient elution) or kept constant (isocratic elution) depending on the complexity of the mixture.
3. Sample Introduction:
– The sample, which contains the mixture of compounds, is dissolved in a small volume of solvent and applied to the top of the column.
– Care is taken to prevent disruption of the stationary phase, ensuring proper flow.
4. Separation Process:
– The sample mixture interacts with both the mobile and stationary phases.
– Components with a strong affinity for the stationary phase adsorb onto the surface and move more slowly, while components with weaker interactions move faster along with the mobile phase.
– Over time, the compounds separate into distinct bands within the column.
5. Detection and Collection:
– As the compounds elute (exit the column), they are collected in fractions. The time or volume at which a compound elutes is called its retention time.
– Detection methods such as UV-visible spectrophotometry, refractive index, or mass spectrometry can be used to identify the compounds as they elute.
6. Column Regeneration:
– After the separation is complete, the column can be cleaned and regenerated for reuse by washing with an appropriate solvent that removes any residual compounds adsorbed on the stationary phase.
Types of Adsorption Chromatography
1. Liquid-Solid Adsorption Chromatography:
– The stationary phase is a solid, and the mobile phase is a liquid. The mixture is dissolved in a solvent and passed through the column, leading to separation based on adsorption.
2. Gas-Solid Adsorption Chromatography:
– The mobile phase is a gas, and the stationary phase is a solid. This method is commonly used for the separation of volatile compounds like hydrocarbons and gases.
Factors Affecting Adsorption Chromatography
– Nature of the adsorbent: Adsorbents like silica gel and alumina provide different polarities, affecting the interaction with the analyte.
– Mobile phase composition: The solvent’s polarity and elution strength influence how quickly compound move through the column.
– Flow rate: A slow flow rate improves resolution but lengthens the process. A faster flow rate may lead to incomplete separation.
– Sample size: Larger samples require more efficient column packing and slower flow rates to maintain resolution.
Advantages of Adsorption Chromatography
1. Wide Range of Applications: Suitable for a variety of organic compounds, drugs, and biological molecules.
2. High Resolution: Capable of separating complex mixtures into individual components with high precision.
3. Reusability: Adsorbents such as silica gel can be regenerated and reused multiple times, making it cost-effective.
4. Scalability: The technique can be used both in analytical and preparative scales.
Disadvantages of Adsorption Chromatography
1. Time-Consuming: The process can be slow, particularly for complex mixtures with multiple components.
2. Adsorbent Deactivation: The stationary phase may lose effectiveness over time due to contamination or degradation after repeated use.
3. Solvent Selection: The wrong choice of solvent can lead to poor separation or degradation of sensitive compounds.
4. Risk of Overloading: Loading too much sample can cause poor separation and band broadening
Applications of Adsorption Chromatography
1. Separation of Natural Products: Used extensively in the purification of plant alkaloids, steroids, and terpenoids.
2. Pharmaceutical Purification:
– Adsorption chromatography is often used in the pharmaceutical industry for the purification of active pharmaceutical ingredients (APIs).
– It’s particularly useful for removing impurities from newly synthesized drugs.
3. Environmental Analysis:
– Used to separate and quantify pollutants, pesticides, and hydrocarbons from environmental samples like air, water, and soil.
4. Organic Chemistry:
– Commonly employed in organic synthesis for the purification and isolation of reaction products.
5. Food Chemistry:
– In food analysis, it is used to separate and identify components like flavor compounds, vitamins, and preservatives.
Elution Techniques in Adsorption Chromatography
1. Isocratic Elution:
– The composition of the mobile phase remains constant throughout the process. It is simpler but may not always give optimal separation for complex mixtures.
2. Gradient Elution:
– The composition of the mobile phase is gradually changed during the process. This technique can enhance the separation of components with very different affinities for the stationary phase.
Common Adsorbents Used
1. Silica Gel (SiO₂):
– The most popular adsorbent, highly polar, and used for the separation of compounds like alcohols, amines, and carboxylic acids.
2. Alumina (Al₂O₃):
– Used for non-polar to moderately polar compounds, particularly for separating aromatic compounds, hydrocarbons, and amines.
3. Activated Charcoal:
– An effective adsorbent for decolorizing solutions and for adsorbing small organic molecules like gases and vapors.
4. Magnesium Oxide:
– Employed for highly polar compounds and often used in the separation of fatty acids and oils.



