Anthracene, a compound derived from coal tar, is known for its presence in coal tar, ranging from 0.3 to 3.5 %. Its name originates from the Greek word “anthrac,” meaning coal. When tar is distilled, anthracene separates into high-boiling fractions known as anthracene oil. Structurally, anthracene comprises three benzene rings fused in ortho positions, resulting in a polycyclic aromatic hydrocarbon. Notably, anthracene appears as a colourless solid.
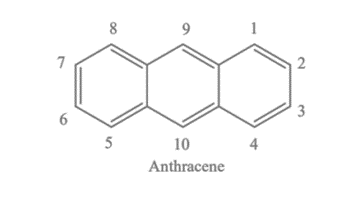
The chemical structure of anthracene consists of three benzene rings fused in a linear arrangement. Each benzene ring contains six carbon atoms arranged in a hexagonal ring, alternating single and double bonds between them. These three benzene rings are fused in anthracene to share carbon atoms along their edges. This results in a polycyclic aromatic hydrocarbon with 14 carbon atoms and 10 hydrogen atoms. The molecular formula of anthracene is C14H10. Its chemical structure can be represented as follows:
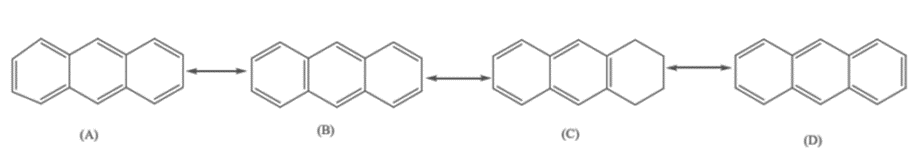
It has sp2 hybridization; the sp2 hybrid orbital overlaps each other and with the s orbital of the ten hydrogen atoms, forming C-C and C-H σ bonds. All carbon bonds in anthracene are not of the same length. in particular, the C1-C2 bond is considerably shorter(1.37A°) than the (C2-C3) bond. (1.42A°). This difference in bond lengths can be understood if we examine the four resonance forms above. Notice that the C1-C2 bond is double in three structures (A, B and C) and single in only one (D), whereas the C2-C3 bond is single in three structures (A, B, and C) and double in only one (D). We would, therefore, expect the C1-C2 bond to have more double-bond characters (shorter bond length) and the C2-C3 bond to have more single-bond characters (longer bond length). The resonance energy of anthracene is 84 kcal/mole. This averages 28 kcal/mole per ring, substantially lower than benzene (36 kcal/mole). As a result, anthracene is much less aromatic than benzene and behaves more like an unsaturated aliphatic hydrocarbon.
Applications:
Anthracene finds various applications across different fields due to its unique properties. Some of its major applications include:
- Dye Production: Anthracene is used in the synthesis of dyes such as alizarin, which is used to produce red and purple dyes.
- Photographic Industry: Anthracene derivatives are employed to manufacture photographic chemicals.
- Organic Synthesis: Anthracene is a starting material in organic synthesis to produce various other compounds.
- Research and Development: Anthracene is frequently used in research laboratories as a model compound in organic chemistry experiments.
- Electronics: Anthracene derivatives have potential applications in organic electronics, including organic light-emitting diodes (OLEDs) and organic photovoltaic cells (OPVs).
- Medicine: Anthracene derivatives have potential medicinal uses, including antibacterial and anticancer properties. Further studies are needed to confirm their efficacy and safety.
- Pyrotechnics: Anthracene is sometimes used to manufacture fireworks and pyrotechnic devices because it produces bright, long-lasting flames when burned.
Anthracene is a versatile compound with various applications in different fields. Its unique chemical structure makes it valuable for use in industries such as dye production, electronics, and research. Its derivatives are used in numerous industrial processes and scientific endeavors, from textiles to pharmaceuticals and organic electronic devices. Anthracene is crucial in advancing technology and innovation and is expected to remain important in research and industry.



