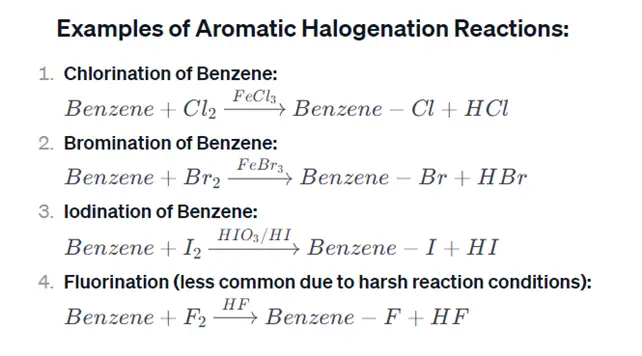
Introduction:
Aromatic halogenation reactions involve the introduction of halogen atoms (fluorine, chlorine, bromine, or iodine) into an aromatic ring. These reactions are a subset of electrophilic aromatic substitution (EAS) reactions, where a halogen replaces a hydrogen atom on the aromatic ring. Here’s an overview of the general mechanism and examples of aromatic halogenation reactions:
Mechanism of Aromatic Halogenation:
1. Generation of Electrophile:
A halogenating agent, often a halogen in the presence of a Lewis acid catalyst, generates an electrophile (halonium ion) capable of attacking the aromatic ring.
2. Attack of Electrophile on Aromatic Ring:
The electrophile attacks the electron-rich aromatic ring. The attack usually occurs at a position with high electron density, commonly ortho or para to existing substituents.
3. Formation of Sigma Complex:
The attack leads to the formation of a sigma complex, an intermediate with one carbon atom temporarily sp3 hybridized.
4. Rearrangement and Loss of a Proton:
A proton is lost from the sigma complex, resulting in a stabilized aromatic intermediate.
5. Regeneration of Aromaticity:
The aromaticity of the ring is restored through resonance stabilization or other processes.
6. Overall Reaction:
The net result is the substitution of a hydrogen atom on the aromatic ring with a halogen.




Outstanding academic content.
Thank you team Pharmacareerinsider.com