Aromaticity in organic chemistry describes the enhanced stability and unique properties of certain cyclic compounds with a specific pi-electron arrangement. The Huckel Rule, formulated by Erich Huckel in the 1930s, provides a quantitative criterion for determining whether a compound exhibits aromatic characteristics.
Key Concepts of Aromaticity:
1. Planarity:
Aromatic compounds are typically planar, with all atoms lying in the same plane. This planarity allows for effective overlap of p-orbitals, facilitating the delocalization of pi-electrons.
2. Conjugation:
Aromatic compounds possess a conjugated pi-electron system. This means there is a continuous chain of alternating single and double bonds or a cyclic system with delocalized pi-electrons. Conjugation contributes to the stability of the system.
3. 4n + 2 Rule:
The Huckel Rule states that a compound is aromatic if it satisfies the 4n + 2 rule, where “n” is an integer (0, 1, 2, 3, …). According to this rule, the number of pi-electrons in the conjugated system must equal 4n + 2, ensuring an odd number of pi-electrons. If the compound fulfills this criterion, it is considered aromatic. It is non-aromatic if it has a conjugated system but does not satisfy the 4n + 2 rule. Compounds with 4n pi-electrons are termed antiaromatic.
Implications of Aromaticity:
1. Stability:
Aromatic compounds are more stable. Then, their non-aromatic counterparts are due to the delocalization of pi-electrons. This stability often manifests in lower reactivity and higher resistance to chemical changes.
2. Unique Reactivity:
Aromatic compounds exhibit unique reactivity patterns. For instance, they are less prone to undergo addition reactions, preferring substitution reactions. This behavior is attributed to the stability of the aromatic ring.
3. Physical Properties:
Aromatic compounds often display distinct physical properties. Such as characteristic UV absorption spectra and enhanced heat of combustion compared to non-aromatic compounds.
Examples of Aromatic Compounds:
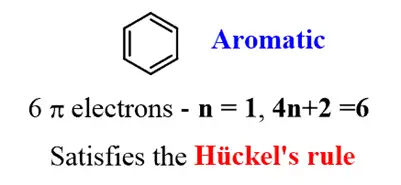
1. Benzene:
The classic example of an aromatic compound, benzene, has a planar hexagonal structure with three alternating double bonds. It satisfies the 4n + 2 rule with six pi-electrons.
2. Pyridine:
Pyridine is a heterocyclic aromatic compound containing a nitrogen atom in the ring. It exhibits aromaticity due to its planarity, conjugated system, and six pi-electrons.
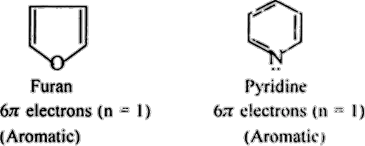
3. Furan:
Furan is another aromatic heterocycle with an oxygen atom in the ring. Its planar structure and conjugated system lead to aromatic behavior with six pi-electrons.
Understanding aromaticity and applying the Huckel Rule is crucial in predicting the stability and reactivity of various organic compounds, providing valuable insights into their behavior in chemical reactions.