Meta Description
Explore benzene and its derivatives, their chemical structure, properties, reactions, and applications in pharmaceuticals, petrochemicals, and organic synthesis.
Introduction
Within the vast expanse of organic chemistry lies the foundational compound known as benzene, a substance whose influence extends through various realms of industrial applications. Resonating at the core of countless synthetic processes, benzene and its derivatives are a testament to human ingenuity and molecular design’s intricate beauty. As we delve into this topic, we will explore the benzene structure to understand its enduring stability, unveil the breadth of benzene uses, and address the health effects associated with its exposure. Join us as we unravel the complexities of this pivotal chemical and its multifaceted implications.
Structure of Benzene and Its Derivatives:

Key Takeaways
- Insight into the unique hexagonal benzene structure and its chemical resonance.
- Comprehensive coverage of benzene uses across diverse industrial sectors.
- An understanding of how the ubiquity of benzene derivatives impacts product development.
- Discussion of benzene health effects to inform safe handling and regulatory compliance.
- Recognition of benzene as a dual character of utility and toxicity within its applications.
The Fundamental Nature of Benzene
Delving into the core elements that define benzene, we find ourselves fascinated by its molecular demeanor. As the epitome of elegance in organic chemistry, benzene’s structure and stability are far more than just a scientific peculiarity—they are pivotal to the compound’s versatile chemical properties.
Exploring Benzene’s Unique Chemical Structure
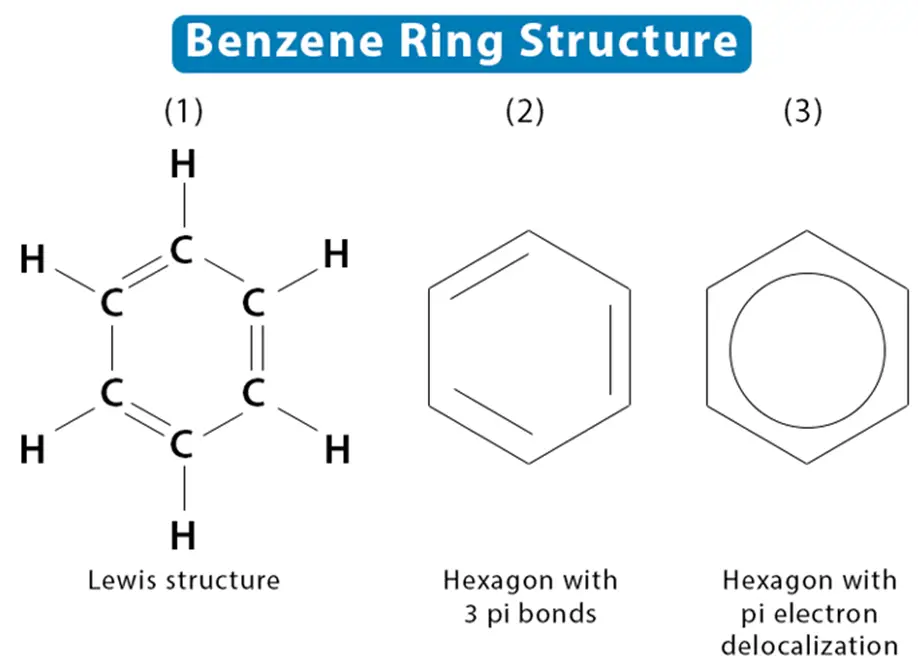
Its distinctive hexagonal ring structure is at the heart of benzene’s reputation in chemistry. The beauty of the benzene structure is in its simplicity and symmetry. Each carbon atom in the benzene ring is bonded to two other carbons and a hydrogen atom, creating a planar ring of six carbon atoms. This elegance is the foundation of benzene’s robust chemical properties.
The Role of Pi Bonds in Benzene’s Stability
The molecule’s resilience is primarily attributed to the presence of pi bonds. Spanning across the entire benzene ring, the pi bonds establish a delocalized system that plays a crucial role in enhancing the molecule’s stability. This configuration prevents the localization of any double bond, rendering benzene resistant to chemical attacks and imparting it with its distinctive inertness. These pi bonds confer a sort of molecular armor against various reactions that typically affect unsaturated hydrocarbons.
Evolution of the Benzene Ring Concept
The journey to elucidate the benzene ring has been a milestone in organic chemistry. Historically, the understanding of the benzene structure has evolved from Kekulé’s alternating double bonds proposal to the more accurate representation of the delocalized pi-electron cloud. This intellectual trek underscores the continued effort to comprehend the depth of the molecule’s chemical properties.
| Feature | Description | Implications for Chemical Properties |
| Hexagonal Ring | Plane of six carbon atoms with alternating single and double bonds. | Facilitates the delocalization of pi electrons, which enhances stability. |
| Pi Bonds | Parallel orbitals above and below the plane of atoms allow shared electrons. | Provides a shield against addition reactions, typical for unsaturated hydrocarbons. |
| Symmetrical Structure | Each carbon bonded identically to adjacent carbons and a hydrogen atom. | Equal distribution of electric charge across the molecule. |
| Delocalized Electrons | Pi electrons are not fixed to a particular pair of carbon atoms; they move freely about the ring. | Contributes to lower reactivity and unique chemical properties. |
As we explore the benzene chemical properties, it’s striking to witness how such a seemingly uncomplicated molecule can unfold into a bastion of stability that has stood the test of time and theory.
Delving into the intricate world of chemical compounds, we find that benzene’s chemical properties play a pivotal role in shaping its reactivity and solubility. These aspects influence benzene’s interaction with other substances and determine its extensive utility across various industrial spheres.
Benzene is renowned for its aromaticity, which grants the molecule a distinct stability and a unique set of reactions in predominantly electrophilic substitution. This reaction is integral to creating a plethora of valuable derivatives that propels benzene’s paramount significance in industrial applications.
To further illustrate, let’s examine the benzene solubility. Benzene is relatively insoluble in water but exhibits high solubility in organic solvents. This property enables benzene to be a versatile solvent, precisely in applications requiring the dissolution of non-polar compounds.
| Solvent | Solubility of Benzene (g/100g of solvent) |
| Water | 0.178 |
| Ethanol | miscible |
| Diethyl Ether | miscible |
| Acetone | miscible |
Our exploration leads us to a keystone reaction of benzene—its benzene reactivity. An example is the aforementioned electrophilic substitution, where benzene acts as a platform for introducing a variety of functional groups, thereby leading to the synthesis of many different compounds that are pivotal in manufacturing drugs, plastics, dyes, and detergents.
- Substitution with a nitro group to form nitrobenzene
- Sulfonation to give benzenesulfonic acid
- Alkylation to produce alkylbenzenes
- Acylation to yield benzene-derived ketones
We discern that benzene’s reactivity and solubility herald its utility in concocting substances that form the underpinning of countless products fundamental to modern life. Therefore, understanding these chemical properties is essential for harnessing benzene’s full potential while mitigating risks associated with its handling and exposure.
Benzene and its Derivatives: A Versatile Group
The realm of organic chemistry is rich with versatile compounds, but few can match the ubiquity and utility of benzene and its derivatives. These substances are common in laboratories and pivotal in many industrial processes. As we delve into this subject, we’ll uncover the roster of common benzene derivatives, the fascinating chemistry they participate in, and their indispensable place in modern industry.
Identifying Common Benzene Derivatives
Certain common benzene derivatives stand out for their widespread use and importance among the sea of aromatic compounds. With its hydroxyl group, Phenol is extensively used as a precursor to plastics and drug compounds. Aniline is a starting point for synthesizing dyes, rubber processing chemicals, and pharmaceuticals. Styrene forms the basis for polystyrene plastics and resins. Each of these derivatives maintains the aromatic ring that is the hallmark of benzene’s structure, modified with functional groups that lend them unique properties.
Chemical Reactions Involving Benzene and Its Substitutes
The chemistry of benzene derivatives is a tapestry of complexity and precision. Substitution reactions, where other atoms or groups replace atoms in the benzene ring, are a cornerstone of benzene chemistry. These reactions often require catalysts to proceed, such as Friedel-Crafts alkylation, which uses aluminum chloride. Hydrogenation, the addition of hydrogen to benzene, turns the liquid hydrocarbon into cyclohexane—a significant feedstock in the manufacture of nylon. Due to its conjugated pi bonds, the benzene ring’s inherent stability makes these reactions highly specific, leading to an array of benzene substitutes tailored for various applications.
Industrial Applications of Benzene Derivatives
The industrial applications of benzene derivatives are vast and diverse, making them a linchpin of many manufacturing sectors. Phenol’s reactivity with formaldehyde produces resins used in plywood and automotive industries. Aniline’s conversion into methylene diphenyl diisocyanate (MDI) lays the groundwork for polyurethane foams, which are critical for insulation and cushioning. Styrene polymerization leads to polystyrene, the lightweight, moldable plastic in packaging, appliances, and consumer goods.
Health and Environmental Impacts of Benzene
The scrutiny of benzene toxicity and its adverse health effects is a subject of paramount importance, bringing to light the severe consequences associated with this widely used chemical. Recognized as a potent carcinogen, benzene represents a dual threat with significant ramifications for human health and the environment. Our focus here extends to detailing how benzene’s presence in our ecosystem necessitates urgent attention and action.
Benzene’s health effects are often long-term and highly detrimental. Exposure, even at low levels, can lead to a spectrum of health issues, particularly blood disorders like anemia and leukemia. The volatile nature of benzene means it can quickly vaporize and penetrate the air we breathe, positioning it as a silent yet formidable hazard.
The pervasive benzene environmental impacts underscore its tenacity and the challenges in containing its influence. When released into the environment—whether through industrial emissions, gasoline fumes, or the improper disposal of products containing this compound—benzene can contaminate water sources, soil, and air. The complexity of its environmental behavior necessitates a multipronged approach to limit its release and mitigate existing contamination.
- Monitoring air quality to detect and manage benzene levels
- Adhering to strict regulations for industries that use benzene
- Developing cleaner technologies to reduce benzene emissions
- Implementing rigorous cleanup operations in contaminated areas
We endeavor to mitigate these impacts, which are multifaceted and ongoing. By integrating stringent regulatory measures and advancing technologies that minimize benzene’s presence in industrial effluents, we aim to shield human health and the environment from this toxic substance. Awareness and vigilance are pivotal in managing the perils posed by benzene, ensuring that safety precedes industrial utility.
The Broad Spectrum of Benzene Uses in Industry
Benzene’s multifaceted role in various industrial sectors underlines its critical importance to modern manufacturing and production processes. Our exploration illuminates the diversity and significance of benzene applications, emphasizing its indispensability and the need for careful management due to associated health risks.
Within the pharmaceutical industry, benzene is vital in creating various drugs that save lives and improve the quality of life. Its chemical properties enable synthesizing complex molecules used as active ingredients in medications, demonstrating its essential nature in health science advancements.
Benzene’s Role in the Manufacture of Plastics and Resins
The manufacturing of plastics and resins heavily relies on benzene. Its derivatives are the building blocks for various polymers and engineering plastics, encompassing widespread use in consumer goods, packaging, automotive components, and other durable materials integral to daily life.
The Importance of Benzene in Petroleum Refining
Petroleum refining processes utilize benzene as a vital intermediate to enhance fuel quality and efficiency. As a component in refining operations, benzene produces cleaner-burning fuels, aligning with global efforts to reduce environmental impact and foster sustainable energy practices.
| Industry Sector | Role of Benzene | Key Products |
| Pharmaceuticals | Drug Synthesis | Antibiotics, Antiseptics, Pain Killers |
| Plastics and Resins | Production of Polymers | PVC, Nylon, Polystyrene |
| Petroleum Refining | Enhancing Fuel Quality | Gasoline, Aviation Fuel, Diesel |
Whether in the development of pharmaceuticals, the sturdy plastics we depend on, or the fuel that powers our vehicles, benzene’s contribution to the industry is profound and pervasive. We recognize benzene’s imperative role across these sectors, reinforcing the substance’s relevance and the collective responsibility to ensure its use is managed with the utmost care for health and ecological considerations.
Benzene Safety Precautions and Handling
In our commitment to safety, we offer robust guidelines for handling benzene and taking diligent benzene safety precautions to prevent exposure. Understanding these measures is critical to ensure workers’ safety and preserve environmental quality. Integrating these protocols into daily operations is essential for minimizing the risks associated with benzene exposure prevention.
Personal Protective Equipment (PPE): Essential for anyone handling benzene, PPE includes respirators, chemical-resistant gloves, goggles, and protective clothing. The aim is to create a barrier between the worker and the hazardous substance, blocking potential entry points.
Proper storage: Benzene should be stored in tightly sealed containers that are clearly labelled and made of materials that resist corrosion. Additionally, these containers should be kept in a well-ventilated area to avoid the accumulation of vapors.
An established and practiced response plan is critical for benzene spill or leak emergencies. Let’s outline the vital components of an emergency response initiative:
- Immediate evacuation of the affected area to minimize exposure.
- Use of spill kits equipped with absorbent materials and protective gear.
- Notify the appropriate emergency response team to manage the incident.
- Decontaminate the spill area and dispose of contaminated materials safely.
| Handling Procedure | Details | Benefits |
| Avoid Direct Contact | Use tools or equipment to handle benzene without direct skin contact. | Reduces the risk of skin absorption and chemical burns. |
| Maintain Adequate Ventilation | Work areas should be equipped with exhaust systems to disperse vapors. | Lowers the concentration of benzene in the air, protecting respiratory health. |
| Regular Monitoring for Leaks | Implement routine inspections of containers and systems for any signs of leaks. | It prevents the accumulation of vapors and mitigates exposure incidents. |
| Proper Disposal | Adhere to guidelines for disposing of benzene waste and contaminated equipment. | Ensures compliance with environmental regulations and protects ecosystems. |
Emphasizing ongoing training and awareness, we bolster our readiness to handle benzene safely. By erecting multiple layers of prevention and response strategies, we fortify our shield against benzene’s dangers, ensuring our collective health and the well-being of our environment.
Understanding Benzene Toxicity and Exposure Risks
As we explore the impact of benzene on public health and safety, it’s essential to deepen our understanding of benzene toxicity and its associated hazards. Benzene, widely used in various industries, poses exposure risks requiring stringent safety precautions. In this section, we’ll analyze benzene’s immediate and long-term health effects, how occupational benzene exposure can be monitored and significantly reduced, and review the existing benzene regulations ensuring employee safety in the workplace.
Acute and Chronic Effects of Benzene on Health
Benzene exposure can lead to both acute and chronic health issues. Short-term exposure may result in dizziness, headaches, and respiratory difficulties. In contrast, long-term exposure is of more significant concern as it can lead to blood disorders like anemia and has been linked to various forms of leukemia. Understanding these health risks is crucial for effectively managing and mitigating benzene exposure risks.
Monitoring and Reducing Occupational Benzene Exposure
In occupational environments where benzene use is prevalent, continuous monitoring is vital. We champion the implementation of real-time air quality assessments and proper ventilation systems to ensure concentrations remain below recommended exposure limits. Additionally, advocating for closed systems to handle benzene can significantly reduce accidental exposure among workers.
Regulations and Standards for Benzene Safety
Various regulations have been established to protect workers and the public from benzene dangers. The Occupational Safety and Health Administration (OSHA) enforces permissible exposure limits, while the Environmental Protection Agency (EPA) regulates air and water standards related to benzene. Below, we’ve outlined these standards, highlighting their roles in benzene safety:
| Agency | Regulation | Permissible Exposure Limit | Monitoring Frequency |
| OSHA | 29 CFR 1910.1028 | 1 PPM | Every six months |
| EPA | Clean Air Act Amendments | N/A | As required |
| NIOSH | Pub. No. 2005-149 | 0.1 PPM | As needed |
| ATSDR | Toxicological Profile for Benzene | N/A | As relevant |
Deconstructing the Benzene Manufacturing Process
The benzene manufacturing process plays a pivotal role in the chemical industry. Our focus is to unfold the intricate details involved in the production of benzene, considering its significant implications on safety, efficiency, and the environment. Distilling the complexities, we aim to elucidate the different methodologies and the latest technologies that make the large-scale production of benzene possible and commercially viable.
In industrial settings, benzene is manufactured through two primary processes: the catalytic reforming of naphtha and the extraction from pyrolysis gasoline derived from ethylene production. Both methods have evolved to meet the growing demand for benzene, optimizing for yield and energy consumption.
- Catalytic Reforming: This process involves the conversion of light hydrocarbons to aromatics through dehydrogenation. Here, benzene is generated alongside other aromatics like toluene and xylenes.
- Steam Cracking: As an integral part of ethylene production, steam cracking produces pyrolysis gasoline, which contains a mixture of aromatic compounds from which benzene can be extracted using various separation techniques.
To enhance our understanding, let’s examine a summarized comparison of the prevalent methods used in benzene production:
| Attribute | Catalytic Reforming | Steam Cracking |
| Feedstock | Naphtha | Hydrocarbons (Ethane, Propane, Naphtha) |
| Main Products | Aromatics (Benzene, Toluene, Xylenes) | Ethylene, Propylene, Pyrolysis Gasoline |
| Byproducts | Hydrogen Gas | Butadiene, Aromatics |
| Technology | Platinum or Rhenium catalysts | High-temperature furnaces quench towers |
| Environmental Consideration | Energy-intensive, Integrated Hydrogen Management | High-temperature operations, Co-product Management |
Bearing the environmental impact in mind, advancements in the benzene manufacturing process continue to emerge, aiming to reduce emissions and improve the sustainability of the production of benzene. It’s our concerted effort to stay informed about these innovations, ensuring that our discussions are anchored in current industrial practices and forward-thinking strategies.
Conclusion
Throughout this exploration of benzene and its derivatives, we’ve illuminated the compound’s indelible impact across various industries, signifying its robust utility and value. Our journey through the structural dynamics and reactivity profiles clarifies how benzene’s distinctive chemical properties underpin its widespread use. From its role in high-stakes sectors like pharmaceuticals and petroleum refining to its presence in the manufacture of everyday plastics, benzene’s derivatives are truly omnipresent in modern society.
Summarizing the Significance of Benzene and Its Derivatives
Emerging Benzene research continues to enhance our understanding of this critical chemical, heightening our appreciation for its versatility and the necessity for caution due to its health risks. By underscoring the chemical’s prominence, we recognize the balance the industry must maintain—leveraging benzene’s benefits while ensuring safety and environmental stewardship.
Future Outlook on Benzene Use and Research
Looking to the horizon, the future of benzene use embodies both promise and challenge. As our reliance on benzene-bearing products persists, so does the imperative to pioneer advancements in its production and application processes. Advancing research in this arena stands pivotal—aiming to minimize health risks while accentuating utility and efficiency.
Encouraging Responsible Management of Benzene-Related Products
In our commitment to progress, we unite in advocating for the responsible management of benzene and its derivatives. Pursuing innovation must pair with conscientious practices and continued stringent safety regulations. Collectively, our surveillance of industry operations and regulatory compliance will stand as guardians over our people’s health and our environment’s sanctity. As custodians of this powerful chemical, our ongoing vigilance and responsibility render the benefits of benzene sustainable for future generations.
FAQ
What is benzene, and what are its primary derivatives?
Benzene is a simple yet foundational aromatic hydrocarbon with the chemical formula C6H6. Its primary derivatives include phenol, aniline, and styrene, derived from benzene through various chemical reactions.
How does the structure of benzene contribute to its chemical stability?
The stability of benzene is attributed to its unique hexagonal ring structure, which includes alternating double bonds and the delocalization of pi electrons across the ring. This configuration results in a stable molecule that is less reactive than predicted for an unsaturated hydrocarbon.
What are the critical health and environmental considerations associated with benzene?
Benzene is classified as a carcinogen and can have severe health effects, including leukemia and other blood disorders. Environmentally, benzene can contaminate air, water, and soil, posing risks to ecosystems. Mitigating exposure and implementing safety controls are, therefore, essential.
In what industries are benzene and its derivatives commonly used?
Benzene and its derivatives are used across various industries, including pharmaceuticals, for drug synthesis, producing plastics, resins, and synthetic materials, and in petroleum refining processes.
What are some safety precautions necessary when handling benzene?
Handling benzene requires strict safety measures, including personal protective equipment (PPE), proper ventilation, exposure limits, emergency response planning for spills or leaks, and regular monitoring to prevent occupational exposure.
How can occupational benzene exposure be reduced?
Reducing occupational benzene exposure involves implementing engineering controls, including closed-system operations and proper ventilation. Strict usage of personal protective equipment is essential. Additionally, adherence to legal exposure limits and regular health surveillance for workers is crucial.
What regulations govern the safe use of benzene in industry?
Organizations like OSHA, EPA, and global regulatory bodies set regulations for safe benzene use. They establish exposure limits, safety guidelines, and monitoring requirements to ensure compliance worldwide.
How is benzene manufactured on a commercial scale?
Commercial-scale benzene is primarily produced through catalytic reforming, toluene hydrodealkylation, and steam cracking of hydrocarbons. These methods focus on converting petroleum derivatives into benzene, requiring careful control to ensure efficiency and minimize environmental impact.