The Bromination of benzene involves the introduction of a bromine atom onto the benzene ring through electrophilic aromatic substitution. A Lewis acid typically catalyzes the reaction, often iron (III) chloride (FeCl3).
Here is the reaction with a concise explanation:

Here is a step-by-step mechanism for the bromination of benzene:
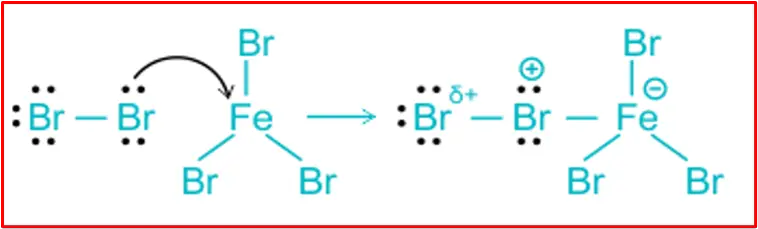
Step 1: Formation of electrophile:
The Lewis acid catalyst (FeCl3) helps generate an electrophile (Cl+) by causing the polarisation of the bromine molecule.
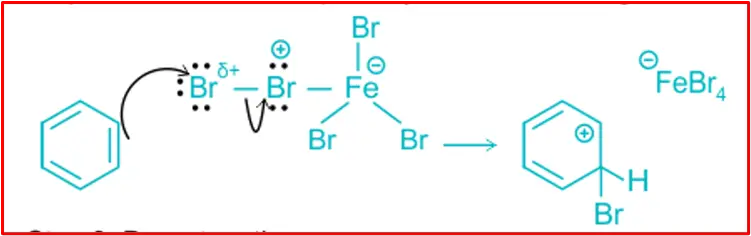
Step 2: Formation of carbocation
The electrophile (Br+) attacks the benzene ring to form an intermediate of carbocation which is resonance stabilised. It is a slow step.
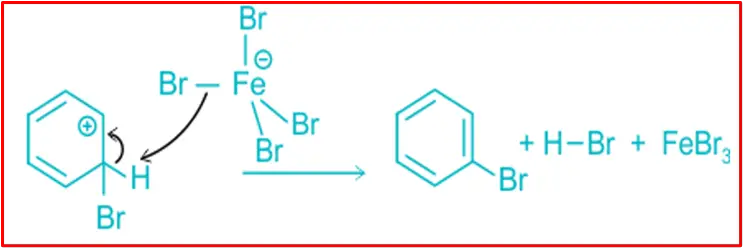
Step 3: Protonation
Proton transfer from the carbocation to form bromobenzene.