Aromatic acids (Carboxylic acids) undergo a variety of chemical reactions due to the presence of the carboxyl group (-COOH) in their structure. Some of the key reactions of carboxylic acids include:
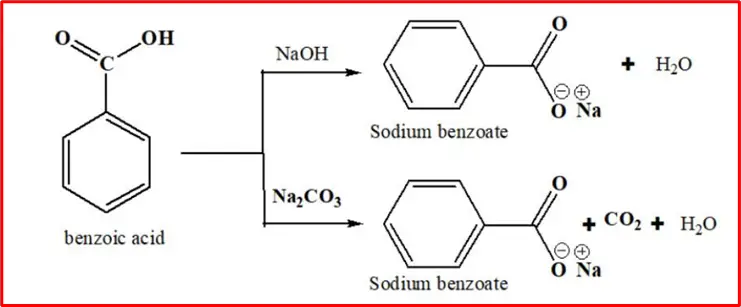
1. Salt formation: When benzoic acid comes into contact with sodium hydroxide or sodium carbonate, it reacts to produce sodium benzoate.
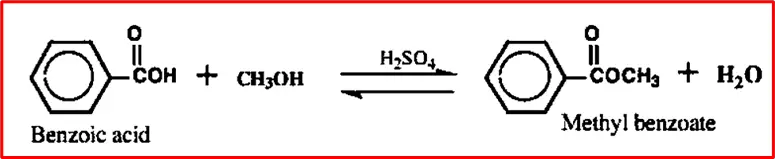
2. Esterification of benzoic acid: When a carboxylic acid and an alcohol react in the presence of a strong acid catalyst, an ester and water are formed. The reaction usually takes place in an alcohol solvent, which is typically present in excess. Sulfuric acid, phosphoric acid, or p-toluene sulfonic acid are commonly used as the acid catalyst.
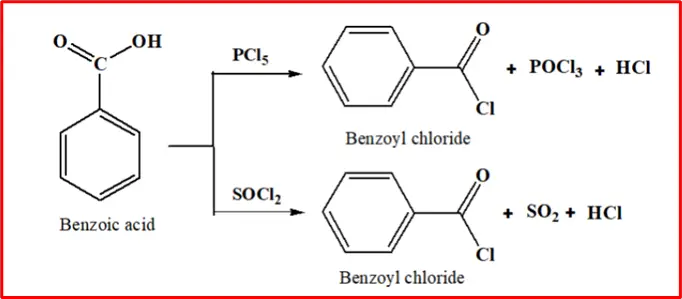
3. Acyl halide formation: When Benzoic acid comes in contact with phosphorus pentachloride or thionyl chloride, it undergoes a reaction to produce benzoyl chloride.
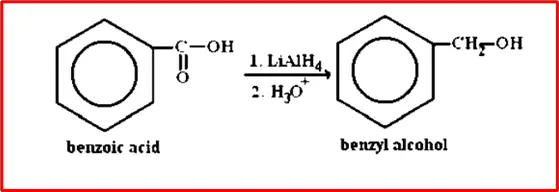
4. Reduction of Benzoic acid to Benzoyl alcohol: Benzoic acid undergoes reduction with lithium aluminium hydride to give benzyl alcohol.
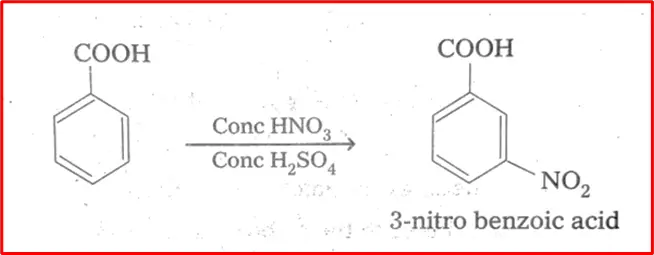
5. Nitration of benzoic acid: Nitration of benzoic acid involves the substitution of one of the hydrogen atoms on the benzene ring with a nitro group (-NO2). The reaction typically requires the use of a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) as the nitrating agent and catalyst, respectively. The reaction proceeds via an electrophilic aromatic substitution mechanism, where the nitronium ion (NO2+) acts as the electrophile.
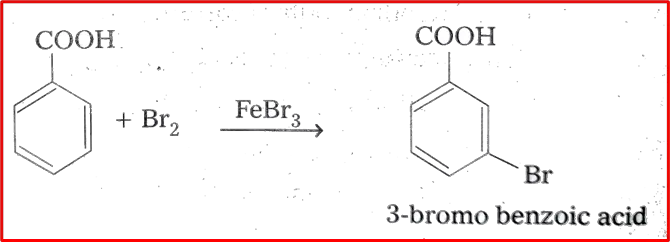
6. Halogenation: The halogenation of benzoic acid is a chemical reaction where a hydrogen atom on the aromatic ring of benzoic acid is replaced with a halogen atom, using the catalyst FeBr3. This reaction usually happens through an electrophilic aromatic substitution (EAS) mechanism that involves the aromatic ring acting as the nucleophile and attacking the electrophilic halogen species.