The chemical reactions of naphthalene are quite similar to those of benzene. It can easily undergo substitution and form additional products. However, it is not as aromatic as benzene. So, the double bonds in naphthalene show some of the reactivity of alkenes, which makes it form additional compounds more quickly than benzene. The reaction stops once one of the rings is fully saturated with hydrogen or halogen.
1. Electrophilic substitution reactions: Naphthalene, like benzene, undergoes electrophilic substitution reactions. Substitution occurs primarily at C1 (α-position). This can be understood if we examine the intermediate carbonium ion. Two resonance forms can be written for the intermediate carbonium ion obtained from the attack at C1 (without involving the other ring). In contrast, only such a form is possible for substitution at C-2. E+ in the following equations represents an electrophile.
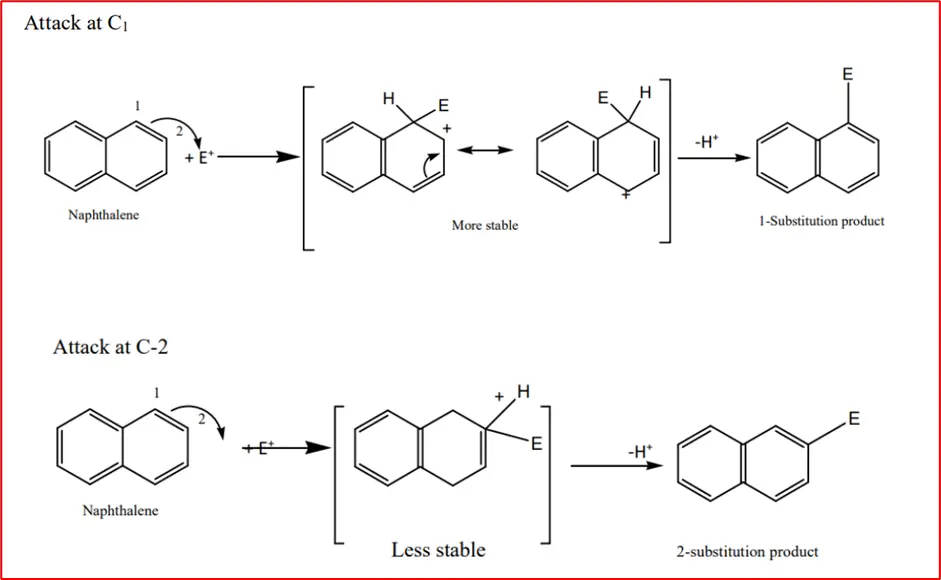
Therefore, the intermediate with the substituent at C-1 is more stable, leading to the predominance of the product with a substituent at C-1. Substitution at C-2 (β-position) only occurs at higher temperatures or with bulkier solvents.
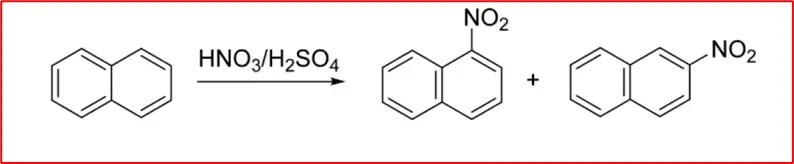
1. Nitration: Naphthalene nitrates with a mixture of nitric acid and sulphuric acid at low temperatures to form mainly the α-nitro naphthalene.
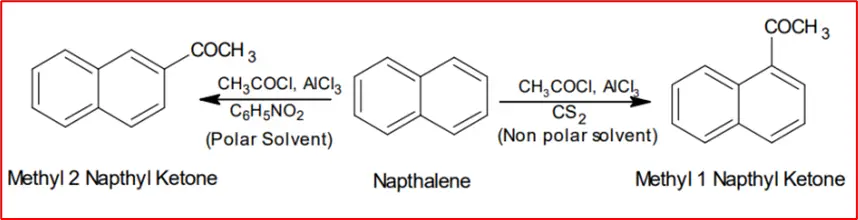
2. Friedal Craft Acylation: Naphthalene reacts with acetyl chloride via Friedel-Crafts reaction to form either the alpha or beta product, depending on the reaction conditions.
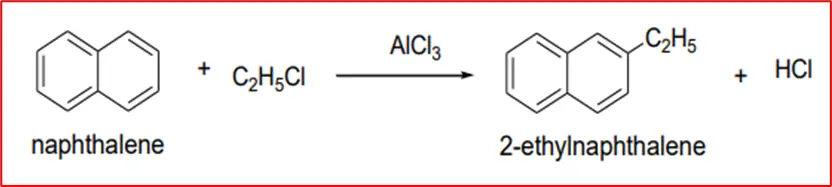
3. Friedal Craft Alkylation: Naphthalene reacts with alkyl halides in the presence of aluminium chloride to form 2-alkyl naphthalene via Friedel-Crafts alkylation.
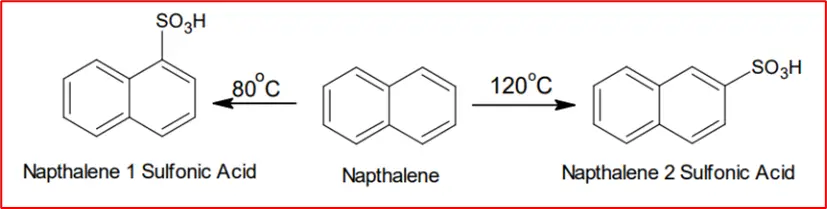
4. Sulphonation: Sulfonation of naphthalene at 80⁰C produces naphthalene-1-sulfonic acid, while at 120⁰C, it produces naphthalene-2-sulfonic acid.
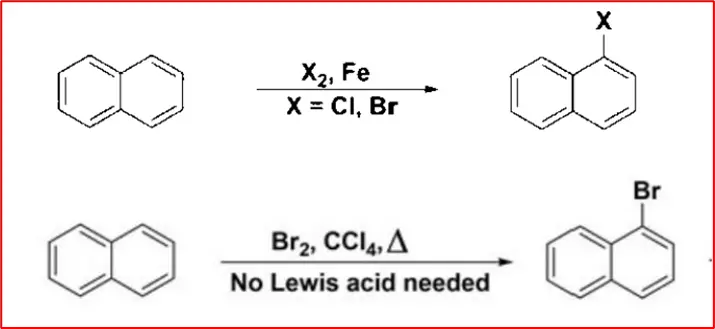
5. Halogenation: Naphthalene reacts with halogen to give the 1-halo derivative of naphthalene.
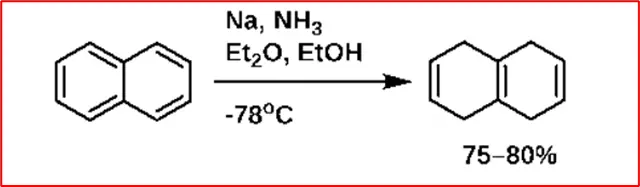
6. Reduction: Naphthalene undergoes reduction more readily than benzene. When it reacts with sodium or ethyl alcohol, it gives 1,4-dihydronaphthalene.
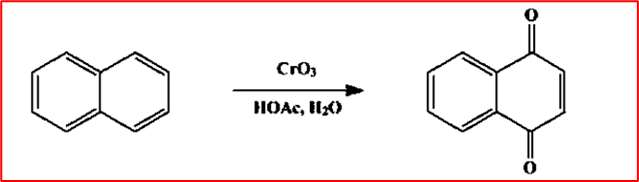
7. Oxidation: When naphthalene is oxidized with chromium trioxide in acetic acid, it produces 1,4-Naphthaquinone.
Conclusion
Naphthalene shares similarities with benzene in its chemical reactivity, favoring substitution reactions predominantly at the C1 position due to the stability of the intermediate carbonium ion. This leads to the formation of various derivatives through processes such as nitration, Friedel-Crafts reactions, sulfonation, halogenation, reduction, and oxidation. These diverse chemical properties highlight the importance of naphthalene in organic chemistry and industrial processes.




