Introduction:
The chlorination of benzene involves the introduction of a chlorine atom onto the benzene ring through electrophilic aromatic substitution. A Lewis acid typically catalyzes the reaction, often iron (III) chloride (FeCl3). Here is the reaction with a concise explanation:
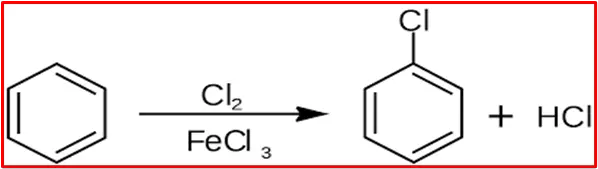
Here is a step-by-step mechanism for the chlorination of benzene:
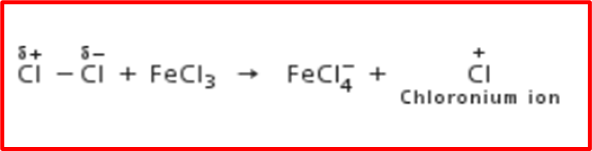
Step 1: Formation of electrophile:
The Lewis acid catalyst (FeCl3) helps generate an electrophile (Cl+) by causing the polarisation of the chlorine molecule.
Step 2: Formation of carbocation
The electrophile (Cl+) attacks the benzene ring to form an intermediate of carbonation, which is resonance stabilized. It is a slow step.
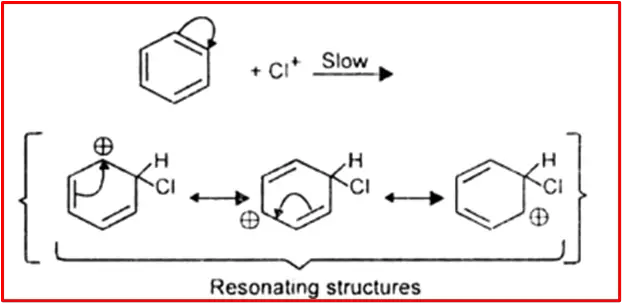
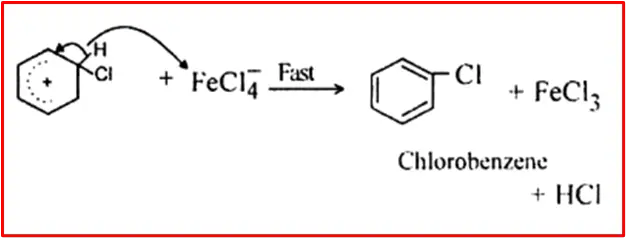
Step 3: Protonation
Proton transfer from the carbocation to form chlorobenzene.