Introduction
Conductometry is an electroanalytical technique used to measure a solution’s electrical conductivity. It is based on the principle that ions in a solution carry an electric current, and the conductivity of the solution depends on the concentration and mobility of these ions. Conductometry is widely used in analytical chemistry to determine the concentration of ionic species, perform titrations, and monitor reaction progress.
Basic Principles of Conductometry
- Conductivity is the ability of a solution to conduct electric current and is influenced by the presence of ions, their concentration, charge, and mobility.
- The electrical conductivity (κ\kappa) of a solution is given by:

- The unit of conductivity is Siemens per meter (S/m) or mho/cm in older units.
- The conductivity of a solution increases with an increase in ion concentration, but at very high concentrations, ion-ion interactions reduce conductivity.
Conductivity Cell
A conductivity cell is a device used to measure the electrical conductivity of a solution. It consists of two electrodes (usually platinum) placed in a solution at a fixed distance apart.
Types of Conductivity Cells
- Dip-Type Cell: Two electrodes are placed parallel and dipped in the solution.
- Flow Type Cell: Used in continuous monitoring systems where the solution flows through the cell.
- Coaxial Type Cell: Electrodes are arranged concentrically for high-precision measurements.
Cell Constant (K)
The cell constant (KK) is defined as:
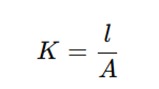
where l is the distance between electrodes and A is the electrode area.
The conductivity (κ\kappa) is related to the measured resistance (R) by:

The cell constant is determined using a standard solution (e.g., KCl solution).
Conductometric Titrations
Conductometric titrations are a type of volumetric analysis where the electrical conductivity of the solution is measured as a function of titrant volume. These titrations are particularly useful when no suitable indicator is available for a traditional titration.
Types of Conductometric Titrations
- Strong Acid vs. Strong Base (HCl vs. NaOH)
- The conductivity decreases as H⁺ ions (high mobility) are replaced by Na⁺ ions (lower mobility).
- After the equivalence point, excess OH⁻ ions increase conductivity.
- Graph: A V-shaped curve.
2. Weak Acid vs. Strong Base (CH₃COOH vs. NaOH)
- Initial conductivity is low as weak acids are poorly ionized.
- Adding NaOH increases ionization, leading to an initial rise in conductivity.
- After equivalence, excess OH⁻ ions cause a sharp rise in conductivity.
3. Strong Acid vs. Weak Base (HCl vs. NH₃)
- Conductivity decreases initially as NH₃ reacts with HCl to form NH₄Cl.
- The weakly ionized NH₄OH formed after the equivalence point results in minimal change.
- Graph: A gradual decrease, then levels off.
4. Weak Acid vs. Weak Base (CH₃COOH vs. NH₃)
- Poorly ionized species result in minimal change in conductivity.
- Graph: A small change in conductivity before and after the equivalence point.
5. Precipitation Titrations (e.g., AgNO₃ vs. NaCl)
- Conductivity decreases as Ag⁺ and Cl⁻ ions form AgCl precipitate.
- After the equivalence point, the addition of excess titrant (AgNO₃) increases conductivity.
- Graph: Decreases and then rises after the equivalence point.
Applications of Conductometry
1. Pharmaceutical and Chemical Analysis
- Determination of drug purity and ion concentration.
- Measurement of total dissolved solids (TDS) in pharmaceutical solutions.
- Monitoring reaction kinetics in chemical synthesis.
2. Environmental Analysis
- Detection of pollutants and heavy metals in water.
- Measurement of salinity in soil and water.
- Monitoring wastewater treatment processes.
3. Industrial Applications
- Quality control in food and beverage industries (e.g., milk conductivity testing).
- Monitoring boiler water purity in power plants.
- Analysis of fuel cell electrolytes.
4. Biological and Medical Applications
- Measurement of electrolyte balance in blood and urine samples.
- Determination of ion content in physiological fluids.
- Development of biosensors for clinical diagnostics.
Advantages of Conductometry
- No need for indicators (useful for colorless or turbid solutions).
- Applicable to weak acids and weak bases where traditional indicators fail.
- Simple, quick, and inexpensive technique.
- Can be automated for continuous monitoring.
Limitations of Conductometry
- Less effective for non-ionic or weakly ionized substances.
- Interference from background electrolytes can affect accuracy.
- It requires calibration and maintenance of conductivity cells.
Conclusion
Conductometry is a powerful electroanalytical technique used for measuring the conductivity of solutions and performing various titrations. It has widespread applications in pharmaceuticals, environmental monitoring, industry, and biomedical sciences. Its simplicity, accuracy, and ability to analyze colorless solutions make it a valuable tool in modern analytical chemistry.


