Coulson and Moffitt’s modification, also known as the bent bond or banana bond model, is a theoretical concept proposed in organic chemistry to explain the stability of certain strained molecules, particularly cycloalkanes. This model builds upon the traditional understanding of bond angles and hybridization to more accurately describe the bonding in cyclic compounds.
In the bent bond model, the carbon-carbon bonds in cycloalkanes are depicted as bent or curved rather than perfectly linear. This bending allows for a redistribution of electron density within the molecule, resulting in greater stability by reducing the strain associated with cyclic structures. The concept of bent bonds helps explain why some cyclic compounds with non-ideal bond angles are still stable and resistant to ring-opening reactions.
What is this Bent bond?
The bent (usually a sigma bonds) have head-on or end-to-end overlapping. The hybrid orbitals, including the intersection bonding region, are present equally on both sides of the molecular axis. But in the case of pi-bonds, the lateral or sidewise overlapping of p-orbitals occurs, and here, the bonding intersection area is totally out of the molecular axis.
What is this Banana bond?
The term “banana bond” is often used to describe the bent configuration of bonds in cyclic molecules, resembling the shape of a banana. This model highlights the importance of considering the spatial arrangement of atoms and electron density distribution in understanding the stability and reactivity of strained organic compounds.
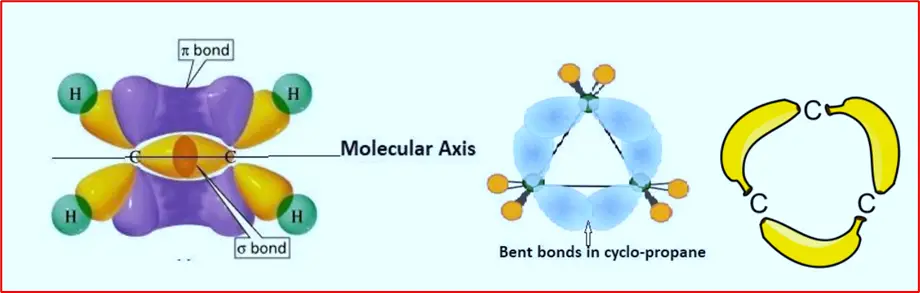
Coulson and Moffitt’s modification provides valuable insights into the structural characteristics of cycloalkanes and other strained molecules, contributing to our understanding of organic chemistry principles and guiding the design of novel molecules with specific properties and functions.




