Introduction
Diazotization titration is a redox titration method used primarily for the quantitative estimation of primary aromatic amines by converting them into diazonium salts using sodium nitrite (NaNO2) in an acidic medium. The technique is widely used in the pharmaceutical, chemical, and dye industries for the determination of aromatic amines in different compounds.
Basic Principles of Diazotization Titration
The principle of diazotization titration is based on the reaction between a primary aromatic amine (R−NH2) and sodium nitrite (NaNO2) in the presence of hydrochloric acid at low temperatures (0-5°C), forming a diazonium salt (R−N2Cl).
General Reaction
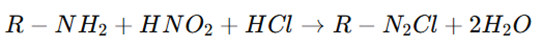
Where:
R−NH2 = Primary aromatic amine
HNO2 = Nitrous acid (generated in situ from sodium nitrite and hydrochloric acid)
R−N2Cl = Diazonium salt
Since diazonium salts are unstable at higher temperatures, carry out diazotization reactions at 0-5°C in acidic conditions to prevent decomposition.
Methods of Diazotization Titration
There are two common methods for performing diazotization titrations:
1. Direct Diazotization Titration
- Used when the aromatic amine forms a stable diazonium salt.
- Cool the sample solution to 0-5°C and gradually add sodium nitrite solution while stirring.
- Detect the endpoint using starch-iodide paper or an external indicator (potassium iodide-starch paper).
- Excess nitrous acid reacts with potassium iodide, releasing iodine, which turns starch paper blue.
2. Back Titration (Indirect Diazotization Method)
- Used when the diazonium salt is unstable or reacts further.
- A known excess of sodium nitrite is added to the amine solution, allowing a complete reaction.
- Titrate the excess sodium nitrite with standard ammonium ceric sulfate or sulfamic acid to determine the amount of unreacted sodium nitrite.
Indicators Used in Diazotization Titration
- Starch-Iodide Paper: Used as an external indicator; it turns blue when excess nitrous acid is present.
- Methyl Orange: Sometimes used as an internal indicator in acidic conditions.
- Ammonium Ceric Sulfate: Used in back titration methods.
Applications of Diazotization Titration
- Pharmaceutical Industry: Used to estimate sulfa drugs (sulfonamides) and procaine (local anesthetics).
- Dye Industry: It aids in the synthesis of azo dyes, which are widely used in textiles and pigments.
- Food Industry: Used to detect and quantify aromatic amines in food preservatives.
- Environmental Analysis: Determines the presence of aromatic amines in industrial wastewater.
- Analytical Chemistry: Used for the quantification of primary aromatic amines in various chemical compounds.
Precautions in Diazotization Titration
- Temperature Control: Always maintain 0-5°C to prevent decomposition of diazonium salts.
- Proper Acid Concentration: Ensure correct acid concentration to avoid side reactions.
- Freshly Prepared Reagents: Freshly prepare sodium nitrite solutions because they degrade over time.
- Proper Handling of Diazonium Salts: These salts are unstable and can decompose explosively at high temperatures.
Conclusion
Diazotization titration is a reliable and accurate method for determining primary aromatic amines in pharmaceuticals, dyes, and environmental samples. Following proper temperature control, reagent preparation, and endpoint detection techniques ensures precise results.