Displacement Value (DV) is a crucial concept in the pharmaceutical field, especially in the preparation of suppositories. It represents the amount of a drug that displaces an equivalent volume of the suppository base. Understanding and calculating displacement values ensures proper dosage and stability of the final formulation.
This article provides a detailed explanation of displacement value, its importance in suppository preparation, and step-by-step calculations to ensure accuracy.
What is displacement value?
Displacement value (DV) is defined as:
“The quantity of drug in grams that displaces 1 gram of the suppository base.”
It determines how much base is replaced when a drug is incorporated into the formulation, ensuring that the final weight remains constant.
Importance of Displacement Value
- Ensures accurate drug dosage in each suppository.
- Prevents overdosing or underdosing, which can affect therapeutic effects.
- Maintains suppository stability by preventing weight and volume variations.
- Essential for proper formulation design in pharmaceutical industries.
How to Calculate Displacement Value
Formula for Displacement Value:
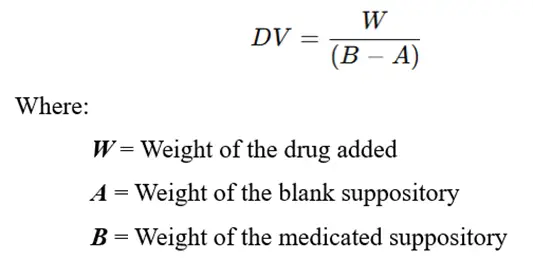
Step-by-Step Calculation Example:
Example 1: Simple Calculation
A pharmacist prepares a batch of suppositories using cocoa butter as the base. The following data is recorded:
- Weight of blank suppository (A) = 2.0g
- Weight of medicated suppository (B) = 1.8g
- Weight of the drug used (W) = 0.5g
Applying the formula:
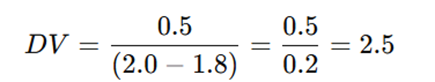
This means that 0.5g of drug displaces 0.2g of the base, and the displacement value of the drug in cocoa butter is 2.5.
Example 2: Calculation with Multiple Ingredients
For a formulation containing two active ingredients, each with different densities and displacement values:
- Calculate the displacement value for each drug individually.
- Adjust the total amount of base accordingly to maintain uniform suppository weight.
If Drug A has a DV of 1.5 and Drug B has a displacement value of 2.0, their combined impact on the base weight must be considered proportionally.
Experimental Method for Determining Displacement Value
- Prepare a series of blank suppositories using the selected base.
- Weigh the blank suppositories and record their average weight.
- Prepare medicated suppositories using a known weight of the drug.
- Weigh the medicated suppositories and calculate the DV using the formula.
Factors Affecting Displacement Value
Several factors influence the displacement value of a drug in a suppository base:
- Density of the drug: Higher-density drugs displace less base.
- Particle size and solubility: Smaller particles distribute more evenly, affecting displacement.
- Nature of the base: Bases like cocoa butter and PEG have different melting points and densities, altering displacement values.
- Temperature: Changes in temperature can affect the consistency and weight of the suppository base.
Common Mistakes in Displacement Value Calculations
Despite its importance, calculating displacement value (DV) is prone to errors, which can impact suppository accuracy and drug efficacy. Below are common mistakes to avoid:
Errors in Weight Measurement
- Improper Balance Calibration: Inaccurate weighing scales can lead to incorrect measurements, affecting the final formulation.
- Moisture Absorption: Some drugs or bases absorb moisture, altering weight readings. Using a desiccator or dry environment is essential.
- Human Error in Weighing Process: Inconsistent handling, especially in manual compounding, can cause variations in displacement calculations.
Impact of Inaccurate Density Estimations
- Assuming Uniform Density: Many formulations assume a uniform drug and base density, but real-world variations exist.
- Ignoring Particle Size and Porosity: Fine vs. coarse drug powders can affect how much base they displace. Precise measurement techniques should be used.
- Temperature-Induced Density Changes: The density of bases like cocoa butter can vary with slight temperature fluctuations, altering the DV.
Advanced Techniques for Displacement Value Determination
To improve accuracy, modern technologies are being used to refine displacement value calculations.
Digital Density Measurement Methods
- Use of Digital Densitometers: These devices accurately measure the density of pharmaceutical compounds, reducing calculation errors.
- Computerized Suppository Formulation Software: Programs like Design of Experiments (DoE) analyze DV variations for optimized formulations.
AI-Driven Suppository Formulation Tools
- Machine Learning Algorithms: AI can predict displacement values based on historical formulation data, reducing the need for repeated experiments.
- Automated Compounding Systems: Robotics and automation ensure precise weighing and blending, minimizing manual errors in DV calculations.
Regulatory Guidelines for Displacement Value Standardization
Ensuring accurate DV calculations is essential for regulatory compliance and product quality.
Pharmacopeial Requirements (USP, BP)
- United States Pharmacopeia (USP): Provides standardized methods for determining DV, ensuring batch consistency.
- British Pharmacopeia (BP): Requires specific quality control tests, including displacement value verification, for suppository formulations.
Industry Best Practices for Quality Control
- Validation of Displacement Value Methods: Pharmaceutical companies conduct validation studies to confirm DV accuracy.
- Routine Batch Testing: Randomized sampling and quality control assessments ensure displacement values remain consistent across production batches.
- Adherence to Good Manufacturing Practices (GMP): Regulatory bodies mandate GMP protocols to maintain product integrity.
Applications of Displacement Value in Pharmaceutical Formulations
- Accurate compounding of suppositories in hospitals and pharmacies.
- Standardization of drug formulations in pharmaceutical industries.
- Optimization of drug release properties by adjusting base and drug interactions.
- Quality control and consistency in manufacturing processes.
Conclusion
Understanding and accurately calculating displacement value is crucial for formulating stable and effective suppositories. By applying proper displacement value calculations, pharmacists and formulators can ensure precise drug dosage and consistent therapeutic outcomes.
Frequently Asked Questions (FAQs)
1. Why is displacement value important in suppository formulation?
Answer: Displacement value ensures correct drug dosage and prevents variations in weight and volume, which are essential for therapeutic efficacy.
2. How does the displacement value vary with different bases?
Answer: Different bases (e.g., cocoa butter, PEG, glycerin) have varying densities and melting behaviors, leading to different displacement values.
3. Can displacement value be calculated theoretically?
Answer: While empirical methods are preferred, theoretical calculations based on density ratios can provide an estimated displacement value.
4. What happens if the displacement value is ignored in the the formulation?
Answer: Ignoring displacement value may lead to overdosing or underdosing, affecting drug stability and patient safety.
5. Are there specific instruments used to determine displacement value?
Answer: The Displacement value is generally determined using weighing balances, suppository molds, and density measurements.



