Introduction
Electrochemical methods of analysis are techniques used to study chemical systems by measuring electrical parameters such as current, voltage, or charge. These methods are widely applied in various fields, including pharmaceutical analysis, environmental monitoring, biomedical research, and industrial applications. Electrochemical analysis is highly sensitive, selective, and capable of detecting trace amounts of substances.
Basic Principles
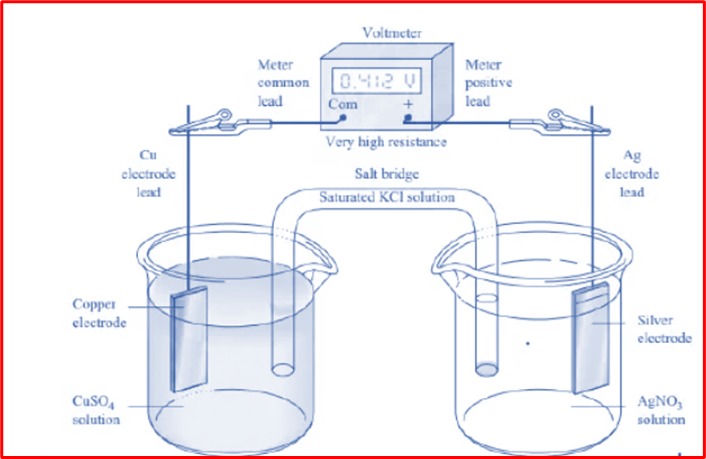
Electrochemical methods are based on redox (oxidation-reduction) reactions occurring at the electrode-solution interface. These methods involve the application of an external potential to drive an electrochemical reaction or the measurement of the electrical responses of a system under controlled conditions.
The fundamental components of an electrochemical system include:
- Working Electrode (WE): Where the reaction of interest occurs.
- Reference Electrode (RE): Maintains a stable potential to measure the potential of the working electrode.
- Counter Electrode (CE): Completes the circuit by allowing current to flow.
- Electrolyte Solution: Contains the analyte and provides an ionic medium for charge transfer.
Types of Electrochemical Methods
1. Potentiometry
- Measures the potential (voltage) of an electrochemical cell under zero current conditions.
- Used for determining ion concentrations using ion-selective electrodes (ISEs), such as pH meters.
- Common electrodes: glass electrode (for pH measurement), Fluoride ion-selective electrode, silver/silver chloride electrode.
2. Voltammetry
Measures current as a function of applied potential.
The analyte undergoes oxidation or reduction at the electrode surface, producing a measurable current.
Types:
Linear Sweep Voltammetry (LSV): Potential is varied linearly with time.
Cyclic Voltammetry (CV): The potential is cycled between two limits to study redox behavior.
Differential Pulse Voltammetry (DPV): Uses small pulses for increased sensitivity.
Square Wave Voltammetry (SWV): Applies square wave pulses to enhance signal detection.
Anodic Stripping Voltammetry (ASV): Used for trace metal analysis.
3. Amperometry
- Measures current at a fixed potential over time.
- Used in biosensors (e.g., glucose sensors) and monitoring oxygen levels.
- Highly sensitive for real-time analysis.
4. Coulometry
- Measures the total charge (coulombs) passed in an electrochemical reaction.
- Two types:
- Controlled Potential Coulometry (CPC): Maintains a constant potential to oxidize/reduce an analyte completely.
- Controlled Current Coulometry: Uses a constant current to determine analyte concentration.
. Highly accurate for determining small amounts of substances.
5. Conductometry
- Measures the electrical conductivity of a solution.
- Used in titrations where ionic strength changes, such as acid-base and precipitation titrations.
- Less sensitive than potentiometry but useful for bulk measurements.
6. Electrogravimetry
- Involves electrodeposition of an analyte on an electrode, followed by weighing the electrode.
- Highly accurate for determining metal content in solutions (e.g., copper, lead).
7. Impedance Spectroscopy (Electrochemical Impedance Spectroscopy, EIS)
- Measures the resistance and capacitance of an electrochemical system.
- Used in battery research, corrosion studies, and biosensor development.
- Provides information on reaction kinetics and diffusion processes.
Applications of Electrochemical Methods
- Pharmaceutical Analysis: Drug purity, content determination, and stability studies.
- Environmental monitoring: detection of heavy metals, pollutants, and pesticides.
- Biomedical Diagnostics: Glucose monitoring (biosensors), DNA sensors, and neurotransmitter analysis.
- Industrial Processes: Quality control in food, beverages, and chemical manufacturing.
- Energy Storage and Conversion: Battery testing, fuel cell analysis, and corrosion studies.
Advantages of Electrochemical Methods
- High sensitivity and selectivity.
- Capable of detecting trace amounts of analytes.
- Fast and cost-effective compared to other analytical techniques.
- Can be used in miniaturized and portable devices (e.g., biosensors).
- It requires a small sample volume.
Limitations
- Some methods require careful calibration and control of experimental conditions.
- Electrode fouling may affect accuracy.
- Interpretation of results may require expertise.
- Sensitivity may be affected by interacting species.
Electrochemical methods of analysis are essential tools in modern analytical chemistry, offering high sensitivity and precision for detecting various substances. These techniques continue to advance, with emerging applications in nanotechnology, biomedical research, and environmental science.