Emulsions are widely used in pharmaceuticals, food, cosmetics, and industrial applications. These mixtures of two immiscible liquids, such as oil and water, require specialized methods to ensure stability and uniformity. The choice of preparation method depends on factors like emulsion type, stability, and intended use.
This article explores the major methods of emulsion preparation and their principles, procedures, and industrial significance.
What is an emulsion?
An emulsion is a mixture of two immiscible liquids, such as oil and water, stabilized by an emulsifier to prevent separation.
Types of Emulsion Preparation Methods
The methods of emulsion preparation can be broadly categorized into mechanical, chemical, and thermal methods. Each method ensures effective dispersion of the dispersed phase within the continuous phase.
1. Mechanical Methods
Mechanical methods utilize physical forces to break and disperse immiscible liquids into each other. These include:
A. High-Speed Mixing (Homogenization Method)
Principle:
High shear force is applied to break down large droplets into fine, uniform-sized particles.
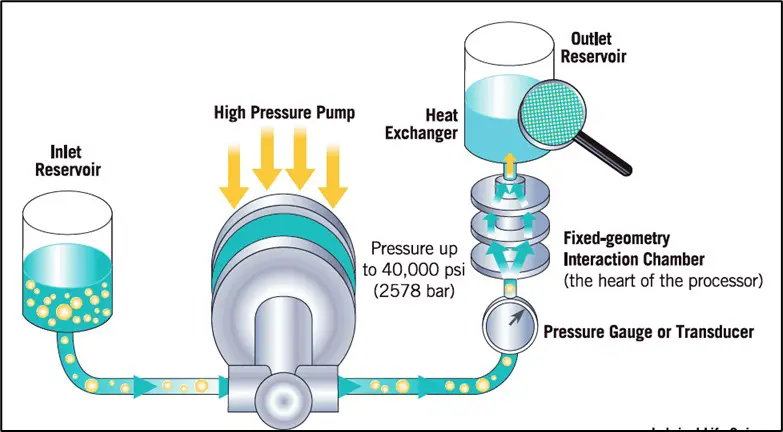
Figure: High-speed homogenization process in emulsion preparation
Procedure:
- The oil and water phases are heated separately.
- Emulsifying agents are added to stabilize the mixture.
- The mixture is subjected to high-speed homogenization using rotor-stator mixers or ultrasonic homogenizers.
- The final emulsion is cooled and stabilized.
Applications:
- Used in pharmaceutical creams, lotions, and dairy products.
B. Mechanical Stirring (Agitation Method)
Principle:
Stirring at a high speed disperses one phase into another, forming an emulsion.
Procedure:
- The oil and water phases are mixed in a beaker.
- A mechanical stirrer agitates the mixture at high speed.
- Emulsifiers are added to enhance stability.
Applications:
- Used in cosmetic creams, mayonnaise, and emulsified sauces.
C. Ultrasonic Emulsification
Principle:
High-frequency ultrasonic waves create cavitation, breaking down droplets into nano-sized emulsions.
Procedure:
- The oil and water mixture is placed in a sonicator chamber.
- Ultrasound waves disrupt the liquid, reducing droplet size.
- The mixture is cooled and stabilized.
Applications:
- Used in nanoemulsions, vaccines, and drug delivery systems.
2. Chemical Methods
Chemical techniques use emulsifying agents or surfactants to stabilize the emulsion.
A. Phase Inversion Method
Principle:
Changing the emulsifier concentration or temperature can invert an oil-in-water (O/W) emulsion to a water-in-oil (W/O) emulsion or vice versa.
Procedure:
- Prepare an O/W emulsion with a low emulsifier concentration.
- Gradually increase emulsifier concentration until the emulsion inverts to W/O.
- Stir continuously to stabilize the new phase.
Applications:
- Used in cosmetics, pharmaceuticals, and food emulsions.
B. Spontaneous Emulsification
Principle:
The rapid diffusion of an organic phase into an aqueous phase forms emulsions without mechanical force.
Procedure:
- A small amount of oil phase is mixed with an organic solvent.
- The mixture is added to water under stirring.
- The solvent evaporates, leaving behind a stable emulsion.
Applications:
- Used in drug delivery formulations.
3. Thermal Methods
Thermal techniques use temperature variations to create emulsions by altering interfacial tension.
A. Heat and Cool Method
Principle:
Heating increases solubility, while cooling stabilizes the emulsion structure.
Procedure:
- The oil and water phases are heated separately (usually 50-70°C).
- The two phases are combined and mixed.
- The mixture is cooled gradually, ensuring uniform droplet dispersion.
Applications:
- Used in lotions, pharmaceutical suspensions, and dairy products.
B. Phase Separation by Cooling
Principle:
A hot mixture forms a homogeneous phase, which separates into an emulsion upon cooling.
Procedure:
- The oil and water mixture is heated until it forms a single-phase solution.
- The mixture is rapidly cooled, causing phase separation into an emulsion.
- Emulsifiers are added to enhance stability.
Applications:
- Used in lipid-based drug delivery and food emulsions.
Advanced Emulsification Techniques
Micro fluidization Method
Principle:
Microfluidization is a high-pressure process that forces immiscible liquids through microchannels to form extremely small and uniform droplets. This method creates nano-sized emulsions with excellent stability.
Procedure:
- The oil and water phases are pre-mixed with an emulsifier.
- The mixture is passed through a high-pressure microfluidizer.
- High shear forces break the droplets into nanoscale sizes.
- The final emulsion is collected and stabilized.
Applications:
- Pharmaceutical nano-emulsions
- Injectable drug formulations
- Cosmetic serums
Membrane Emulsification
Principle:
A porous membrane with uniform-sized pores allows controlled droplet formation when one phase is forced through the membrane into another immiscible phase.
Procedure:
- The oil or water phase is forced through the membrane.
- Uniform-sized droplets are created as they pass into the continuous phase.
- Stirring or mixing ensures dispersion and stability.
Applications:
- Encapsulation of nutrients
- Drug delivery systems
- Food emulsions
Self-Emulsifying Drug Delivery Systems (SEDDS)
Principle:
SEDDS utilize a mixture of oils, surfactants, and co-solvents to form emulsions spontaneously when mixed with an aqueous medium, improving drug solubility and bioavailability.
Procedure:
- Lipophilic drugs are dissolved in oil with surfactants.
- The formulation is filled into capsules or added to liquid dosage forms.
- Upon oral administration, it self-emulsifies in the gastrointestinal tract.
Applications:
- Oral drug delivery
- Lipid-based drug formulations
Factors Affecting Emulsion Preparation
pH and Ionic Strength
- The pH of the system affects the charge on emulsifier molecules, influencing stability.
- Electrolytes (salts) can destabilize emulsions by altering interfacial tension and droplet interactions.
- Adjusting pH helps maintain stability, especially in pharmaceutical and cosmetic emulsions.
Temperature Control
- Higher temperatures reduce viscosity, allowing better droplet dispersion.
- Excessive heat can degrade heat-sensitive compounds, requiring precise control.
- Temperature-sensitive emulsions use controlled cooling methods to enhance stability.
Shear Force & Equipment
- High-pressure homogenizers: Best for pharmaceutical and nano-emulsions.
- Rotor-stator mixers: Suitable for creams and food emulsions.
- Ultrasonic processors: Used for fine nano-emulsions in drug delivery.
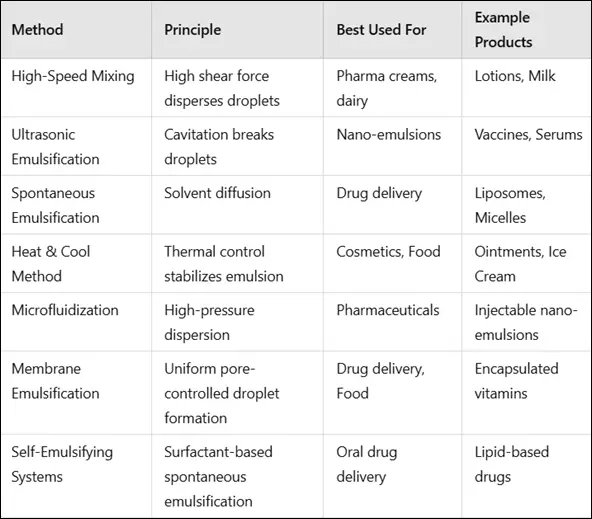
Comparison Table of Emulsion Preparation Methods
Common Emulsification Challenges & Solutions
Phase Separation
Issue: Droplets merge, leading to separation of oil and water.
Solution: Use strong emulsifiers like lecithin or polysorbates, and apply high shear mixing to create stable droplets.
Creaming vs. Sedimentation
- Creaming: Lighter droplets rise due to density differences.
- Sedimentation: Heavier droplets settle.
- Solution: Adjust viscosity using thickening agents (gums, polymers) and optimize droplet size through homogenization.
pH Sensitivity
Issue: Changes in pH affect emulsifier charge, leading to instability.
Solution: Use buffer systems to maintain optimal pH and stabilize emulsifiers.
Factors Affecting Emulsion Preparation
Several factors influence the stability and efficiency of emulsion formation:
- Emulsifier type and concentration
- Temperature of preparation
- Shear force applied
- Droplet size distribution
- pH and ionic strength of the medium
Industrial Applications of Emulsion Preparation Methods
Different industries use emulsion preparation methods to enhance product performance:
- Pharmaceutical Industry: Creams, ointments, vaccines
- Food Industry: Salad dressings, ice creams, sauces
- Cosmetic Industry: Lotions, hair care, makeup formulations
- Paint & Coating Industry: Water-based paints, coatings
Conclusion
Choosing the right emulsion preparation method depends on the desired stability, droplet size, and application. Mechanical, chemical, and thermal methods each offer unique benefits for various industries. Understanding these techniques helps manufacturers develop high-quality emulsions for pharmaceuticals, food, and cosmetics.
Frequently Asked Questions (FAQs)
1. Which method is best for preparing stable emulsions?
Answer: The homogenization method is widely used for creating stable emulsions with fine droplet sizes.
2. How does phase inversion affect emulsions?
Answer: Phase inversion alters the type of emulsion, changing it from O/W to W/O or vice versa, affecting stability and performance.
3. Why are emulsifying agents necessary?
Answer: Emulsifying agents reduce surface tension and prevent phase separation, ensuring long-term emulsion stability.
4. What role does temperature play in emulsion formation?
Answer: Temperature affects viscosity, interfacial tension, and solubility, influencing emulsion stability.
5. Can emulsions be prepared without surfactants?
Answer: Yes, some spontaneous emulsification methods do not require surfactants but rely on phase separation principles.



