What are emulsions?
Emulsion stability is crucial in the pharmaceutical, food, cosmetic, and chemical industries. Discover common causes of instability and effective strategies to enhance emulsion shelf life and performance.
Importance of Emulsion Stability in Different Industries
Emulsion stability plays a crucial role in various industries, ensuring product quality, efficacy, and safety. Below are industry-specific examples:
- Pharmaceuticals: Intravenous emulsions and topical creams must remain stable to ensure proper drug delivery and bioavailability. Unstable emulsions can lead to phase separation, reducing therapeutic effectiveness.
- Food Industry: Emulsified products like mayonnaise, salad dressings, and dairy-based beverages require stability to maintain texture, taste, and shelf life. Separation can lead to spoilage and consumer dissatisfaction.
- Cosmetics: Lotions, creams, and serums rely on stable emulsions for smooth application and absorption. Instability can cause uneven distribution of active ingredients.
- Chemical Industry: Industrial emulsions such as paints, coatings, and lubricants need stability to prevent phase separation and maintain performance over time.
Ensuring stability in these industries not only improves product quality but also reduces production costs and waste.
Common Stability Problems in Emulsions
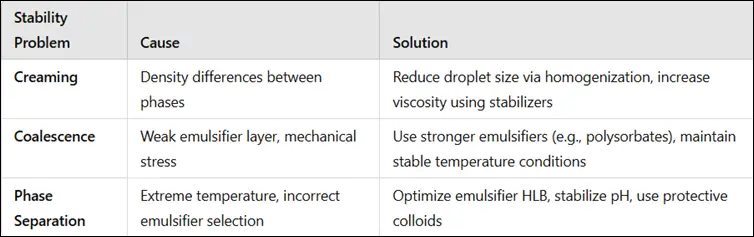
1. Creaming
Description:
- Creaming occurs when dispersed phase droplets rise to the top or settle at the bottom due to density differences.
- The emulsion appears non-uniform, leading to inconsistency in performance.
Causes:
- Density difference between dispersed and continuous phases.
- Large droplet size.
- Insufficient viscosity of the continuous phase.
Methods to Overcome:
- Reduce droplet size by high-speed homogenization.
- Increase the viscosity of the continuous phase using thickening agents like xanthan gum or gelatin.
- Use density modifiers to balance the phases.
2. Flocculation
Description:
- Flocculation occurs when dispersed phase droplets come together but do not merge.
- Leads to increased droplet size and possible coalescence.
Causes:
- Inadequate emulsifier concentration.
- Insufficient electrostatic repulsion between droplets.
Methods to Overcome:
- Use ionic emulsifiers to create electrostatic repulsion.
- Optimize emulsifier concentration to ensure adequate droplet protection.
- Adjust pH and ionic strength to maintain charge stabilization.
3. Coalescence
Description:
- Coalescence is the merging of small droplets into larger ones, leading to phase separation.
Causes:
- Insufficient emulsifier coverage on droplets.
- Mechanical stress (e.g., shaking, temperature fluctuations).
Methods to Overcome:
- Use stronger emulsifiers like polysorbates or phospholipids.
- Increase interfacial film strength by using co-emulsifiers.
- Maintain stable temperature conditions.
4. Phase Separation (Breaking)
Description:
- Complete separation of the two phases, leading to product failure.
Causes:
- Incorrect emulsifier selection.
- Extreme temperatures.
- High electrolyte concentration.
Methods to Overcome:
- Select an appropriate HLB (hydrophilic-lipophilic balance) emulsifier.
- Maintain stable temperature and pH conditions.
- Protective colloids (e.g., polymers, proteins) strengthen interfacial layers.
5. Ostwald Ripening
Description:
- Gradual growth of larger droplets at the expense of smaller ones, leading to instability.
Causes:
- Solubility differences between droplets of varying sizes.
- High interfacial tension.
Methods to Overcome:
- Use insoluble oils to reduce Ostwald ripening.
- Add antioxidants and surfactants to slow down droplet diffusion.
- Reduce interfacial tension by optimizing emulsifier concentration.
6. Inversion of Emulsion Type
Description:
- An oil-in-water (O/W) emulsion changes to a water-in-oil (W/O) emulsion or vice versa.
Causes:
- High shear mixing.
- Temperature fluctuations.
- Change in emulsifier concentration.
Methods to Overcome:
- Control shear force during preparation.
- Use temperature-stable emulsifiers.
- Maintain an optimal HLB value.
Advanced Techniques to Improve Emulsion Stability
Nanoemulsions
- Smaller droplet sizes enhance long-term stability.
- Provides better bioavailability in pharmaceuticals and improved texture in food and cosmetics.
Microfluidization
- High-pressure homogenization technique for stable emulsions.
- Reduces droplet size and improves uniformity.
Layered Emulsifiers
- Multi-layer stabilization using polysaccharides, proteins, and surfactants.
- Enhances resistance against coalescence and Ostwald ripening.
Role of Surfactants & Emulsifiers in Stability
Hydrophilic-Lipophilic Balance (HLB)
- Determines emulsifier efficiency for oil-in-water vs. water-in-oil emulsions.
Best Emulsifiers for Different Industries
- Pharmaceuticals: Lecithin, polysorbates.
- Food: Egg yolk, soy lecithin.
- Cosmetics: Phospholipids, glycolipids.
- Chemical: Silicone emulsifiers.
Natural vs. Synthetic Emulsifiers
- Natural emulsifiers (e.g., lecithin, gum arabic) are sustainable but may lack stability.
- Synthetic emulsifiers (e.g., polysorbates) offer better long-term stability.
Preventive Strategies for Long-Term Emulsion Stability
- Optimize Emulsifier Concentration: Ensure the correct ratio of emulsifier to dispersed phase.
- Control pH and Ionic Strength: Adjusting these factors prevents droplet aggregation.
- Maintain Proper Storage Conditions: Keep emulsions at stable temperatures and avoid mechanical stress.
- Use stabilizers and co-emulsifiers: They enhance interfacial film strength and prolong stability.
- Regular Quality Testing: Conduct particle size analysis, zeta potential measurement, and viscosity testing for quality control.
Conclusion
Emulsion stability is a critical factor that influences product efficacy across various industries. Manufacturers can produce high-quality, long-lasting emulsions by understanding and addressing common instability issues such as creaming, flocculation, coalescence, phase separation, Ostwald ripening, and inversion. Implementing advanced stabilization techniques, appropriate emulsifier selection, and maintaining ideal processing conditions can significantly enhance emulsion performance and shelf life.
Frequently Asked Questions (FAQs)
1. What is the most common cause of emulsion instability?
Answer: The most common causes include inadequate emulsifier concentration, phase separation, and external stress factors like temperature fluctuations.
2. How can I prevent creaming in an emulsion?
Answer: Creaming can be prevented by reducing droplet size, increasing viscosity, and balancing the density of the dispersed and continuous phases.
3. Why does an emulsion break over time?
Answer: Emulsions break due to coalescence, phase inversion, or extreme environmental conditions like heat or freezing.
4. What role do emulsifiers play in emulsion stability?
Answer: Emulsifiers stabilize emulsions by reducing interfacial tension, preventing droplet merging, and enhancing electrostatic repulsion.
5. Can storage conditions affect emulsion stability?
Answer: Yes, temperature changes, exposure to light, and mechanical agitation can all affect the stability of emulsions.