Introduction
Gravimetric analysis is one of the most accurate and precise quantitative analytical techniques. Estimating barium sulfate (BaSO₄) is a classic example of this method, used to determine the sulfate ion (SO42−) content in a given sample. The principle is based on precipitating sulfate ions as insoluble barium sulfate, followed by filtration, drying, and weighing to determine the sulfate concentration.
Applications
- Water analysis: To determine sulfate contamination in drinking water.
- Pharmaceutical industry: To analyze sulfate-containing drugs and formulations.
- Fertilizer industry: To measure sulfate content in fertilizers.
- Environmental analysis: To check sulfate pollution in industrial wastewater.
Principle
The estimation of sulfate is based on the precipitation reaction between sulfate ions (SO42−) and barium chloride (BaCl2), forming insoluble barium sulfate (BaSO4), which is then isolated and weighed gravimetrically.

This reaction occurs under acidic conditions to prevent interference from carbonate or phosphate impurities. The formed precipitate is a fine, white crystalline solid, which is filtered, washed, dried, and weighed for sulfate quantification.
Procedure for Estimation of Barium Sulfate
1. Preparation of Sample Solution
- Weigh a known amount of the sulfate-containing sample (e.g., sodium sulfate or a sulfate-containing compound).
- Dissolve the sample in distilled water in a beaker.
- Add a few drops of dilute hydrochloric acid (HCl) to prevent co-precipitation of other ions like carbonate (CO32−) or phosphate (PO43−).
2. Precipitation of Barium Sulfate
- Heat the solution to 70-80°C to improve crystal growth and reduce impurities.
- Add barium chloride (BaCl2) solution dropwise while stirring continuously.
- A white precipitate of barium sulfate (BaSO4) forms immediately.
- Allow the precipitate to stand for 15-30 minutes to ensure complete precipitation.
3. Digestion (Ostwald Ripening)
- The precipitate remains in the hot solution for about 30–60 minutes, allowing smaller particles to rearrange and form larger, purer crystals.
- This step helps remove any trapped impurities from the precipitate.
4. Filtration and Washing
- Filter the precipitate using a pre-weighed ashless filter paper or sintered glass crucible.
- Wash the precipitate several times with hot distilled water to remove excess BaCl₂ and soluble impurities.
- Wash carefully to prevent the loss of the precipitate.
5. Drying and Ignition
- Dry the filtered precipitate in an oven at 100-120°C to remove moisture.
- If necessary, ignite the precipitate in a muffle furnace at 800-900°C to remove any organic contaminants.
- Cool the sample in a desiccator before weighing it to prevent moisture absorption.
6. Weighing and Calculation
- Weigh the dried barium sulfate precipitate accurately using an analytical balance.
- The amount of sulfate ion (SO42−) in the sample is calculated using the formula:
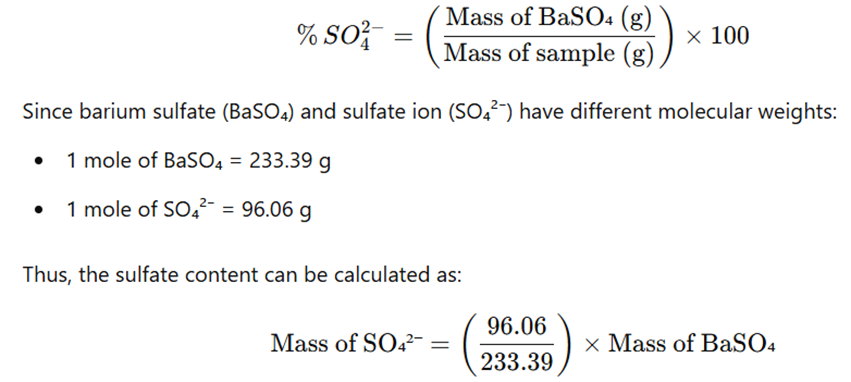
Sources of Error and Precautions
- Slow and controlled precipitation ensures larger, purer crystals.
- Heating the solution to 70-80 °C prevents the formation of colloidal precipitate.
- Proper washing of the precipitate removes contamination from soluble salts.
- Excess barium chloride (BaCl2BaCl_2) should be avoided to prevent co-precipitation errors.
- Using dilute HCl helps prevent interference from carbonate or phosphate precipitation.
- Proper drying and ignition ensure the complete removal of moisture and volatile impurities.
Conclusion
The gravimetric estimation of barium sulfate is a highly precise and reliable method for determining sulfate concentration in a sample. By following proper precipitation, digestion, filtration, drying, and weighing techniques, errors can be minimized, leading to accurate analytical results.




