Objective:
To estimate the amount of calcium gluconate in a given sample using complexometric titration with EDTA as the titrant.
Principle:
In this titration, calcium ions (Ca2+) react with EDTA (ethylenediaminetetraacetic acid) to form a stable 1:1 complex. The endpoint is detected using an indicator, usually Murexide or Eriochrome Black T, which changes color when all calcium ions have reacted with EDTA.
Requirements:
Reagents:
- Standard EDTA solution (0.01 M or appropriate concentration).
- Murexide indicator (or Eriochrome Black T).
- Buffer solution (pH 10): Ammonium chloride-ammonia buffer.
- Calcium gluconate solution (unknown concentration).
- Distilled water.
Apparatus:
- Burette.
- Pipette.
- Conical flask.
- Beaker.
- Measuring cylinder.
Procedure:
1. Preparation of Reagents
EDTA Solution: Prepare a 0.01 M EDTA solution by dissolving 3.7224 g of disodium EDTA in 1 L of distilled water.
Buffer Solution (pH 10): Prepare by mixing ammonium chloride and ammonia in a 1:1 ratio and adjusting the pH to 10 using dilute ammonia.
Indicator Solution:
– Murexide is prepared by dissolving 0.2 g in 100 mL of distilled water.
– For Eriochrome Black T, prepared by dissolving 0.5 g in 100 mL of ethanol or water.
2. Preparation of Calcium Gluconate Solution
- Accurately weigh a known amount of calcium gluconate sample (e.g., 1 g).
- Dissolve the sample in a small volume of distilled water.
- Transfer to a 250 mL volumetric flask and dilute to the mark with distilled water.
3. Titration
- Pipette 25 mL of the calcium gluconate solution into a clean conical flask.
- Add 5 mL of buffer solution (pH 10) to maintain the required pH for the reaction.
- Add a pinch of Murexide indicator (or 3-4 drops of Eriochrome Black T).
– If using Murexide, the solution turns pink.
– If using Eriochrome Black T, the solution turns wine-red. - Fill the burette with the standard EDTA solution.
- Titrate the solution with EDTA while swirling the conical flask until the color changes:
– For Murexide, the color changes from pink to purple.
– For Eriochrome Black T, the color changes from wine-red to blue. - Note the burette reading at the endpoint.
4. Repeat Titration
- Repeat the titration 2-3 times for concordant readings.
- Record the readings and calculate the average volume of EDTA used.
Calculation
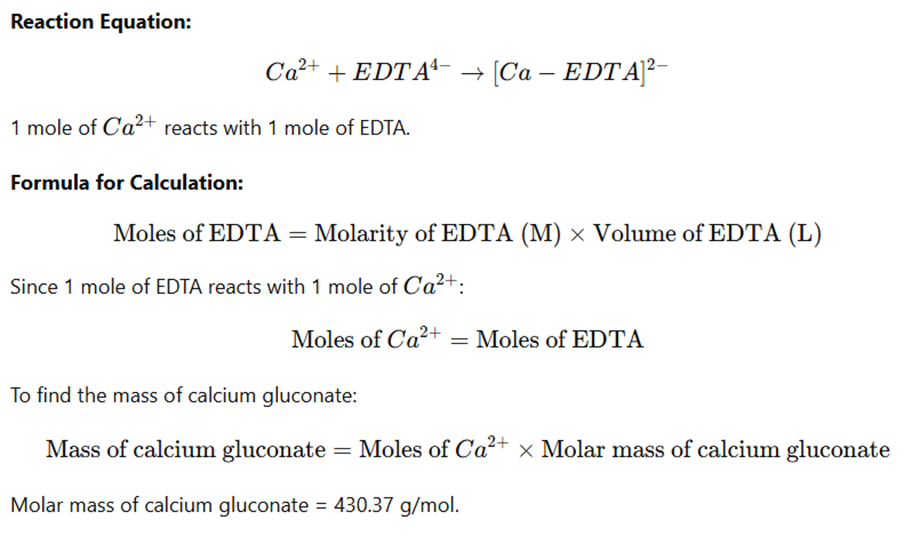
Example Calculation:
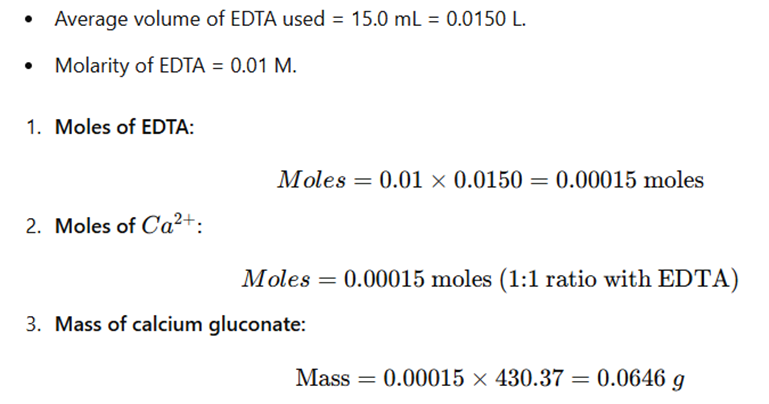
Results:
The amount of calcium gluconate in the sample is 0.0646 g per 25 mL of solution.




