Introduction
Sodium chloride (NaCl) is crucial in various fields such as pharmaceuticals, food, water analysis, and chemical industries. Its estimation is commonly performed using precipitation titration methods such as Mohr’s, Volhard’s, or Fajan’s methods. These methods involve titration of chloride ions (Cl−) with silver nitrate (AgNO3), forming a precipitate of silver chloride (AgCl).
Methods for Estimating Sodium Chloride
Three main methods are used for the quantitative determination of NaCl:
- Mohr’s Method (Direct Precipitation Titration using Chromate Indicator)
- Volhard’s Method (Back Titration using Ferric Indicator in Acidic Medium)
- Fajan’s Method (Adsorption Indicator Method)
1. Mohr’s Method (Direct Titration)
Principle
- Chloride ions (Cl−) from sodium chloride react with silver nitrate (AgNO3) to form a white precipitate of silver chloride (AgCl).
- A few drops of potassium chromate (K2CrO4) are added as an indicator.
- When all the chloride ions have reacted, excess silver ions (Ag+) react with the chromate to form red silver chromate (Ag2CrO4), indicating the endpoint.
Reaction

Procedure
- Prepare a standard AgNO₃ solution (0.1 N).
- Pipette a known volume of NaCl solution into a conical flask.
- Add a few drops of potassium chromate (K2CrO4) indicator.
- Titrate with AgNO₃ solution until a reddish-brown color appears (formation of silver chromate).
- Record the volume of AgNO₃ used and calculate the NaCl concentration.
Calculation
Using the normality equation:

Limitations
- It must be carried out at neutral pH (7-10) to prevent interference.
- The presence of bromide or iodide may affect accuracy.
2. Volhard’s Method (Back Titration)
Principle
- This method adds excess standard silver nitrate (AgNO₃) to precipitate all chloride ions (Cl−) as silver chloride (AgCl).
- The process then back-titrates the unreacted silver ions with standard potassium thiocyanate (KSCN) using ferric ammonium sulfate (Fe³⁺) as an indicator.
- At the endpoint, red ferric thiocyanate (Fe(SCN)3) forms, marking the completion of the reaction.
Reaction
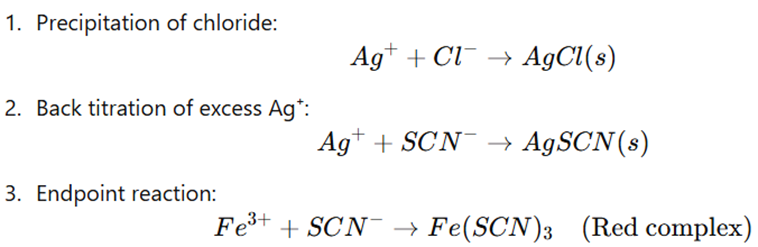
Procedure
- Add excess AgNO₃ to the NaCl solution and allow complete precipitation.
- Filter and remove the AgCl precipitate.
- Back titrate the remaining Ag⁺ ions with standard KSCN solution, using ferric ammonium sulfate as an indicator.
- The appearance of a red ferric thiocyanate complex indicates the endpoint.
Calculation
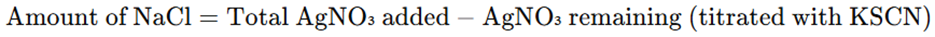
Advantages
- Suitable for colored or turbid solutions.
- Can be used for acidic solutions (pH 2-3).
Limitations
- Requires filtration, which makes the process time-consuming.
3. Fajan’s Method (Adsorption Indicator)
Principle
- Adding silver nitrate (AgNO₃) to a sodium chloride solution forms a precipitate of silver chloride (AgCl).
- The process uses an adsorption indicator (fluorescein or eosin).
- Initially, the precipitate has a negative charge due to excess Cl⁻ ions.
- At the equivalence point, the charge reverses to positive (due to excess Ag⁺ ions).
- The negatively charged adsorption indicator binds to the precipitate and undergoes a color change, marking the endpoint.
Reaction

Procedure
- Add fluorescein or eosin indicator to the NaCl solution.
- Titrate with AgNO₃ while stirring.
- A color change detects the endpoint (e.g., fluorescein changes from yellow-green to pink).
Advantages
- Highly sensitive method.
- No filtration is necessary.
- Works for dilute solutions.
Limitations
- pH-sensitive; requires neutral or slightly acidic conditions.
- Only suitable for specific ions like Cl⁻, Br⁻, I⁻.
Comparison of Methods
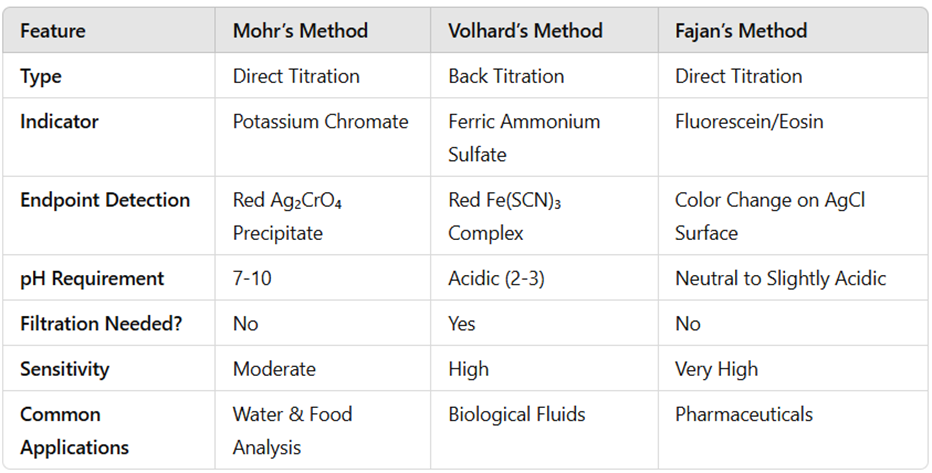
Conclusion
You can estimate sodium chloride using Mohr’s, Volhard’s, or Fajan’s method, depending on the sample conditions and the required accuracy. Mohr’s method is the simplest, Volhard’s method is ideal for acidic solutions, and Fajan’s method provides the highest sensitivity. Each technique has its advantages and limitations, making it essential to choose the appropriate method based on the sample characteristics.



