Semi-solid dosage forms, such as ointments, creams, gels, and pastes, are widely used in topical and transdermal drug delivery. Ensuring their quality, stability, and therapeutic efficacy requires rigorous evaluation through various physicochemical, rheological, and microbiological tests.
This article explores the key methods and parameters used to evaluate semi-solid pharmaceutical formulations.
What are semi-solid dosage forms?
Semi-solid dosage forms are pharmaceutical formulations with properties between solid and liquid states. These include:
- Ointments: Oil-based formulations providing occlusive effects.
- Creams: Emulsions of water and oil, offering moisturizing effects.
- Gels: Transparent formulations with gelling agents for enhanced drug absorption.
- Pastes: Highly viscous formulations containing a high percentage of insoluble solids.
Each type has distinct pharmaceutical applications based on absorption, occlusion, and drug release characteristics.
Key Evaluation Parameters of Semi-Solid Dosage Forms
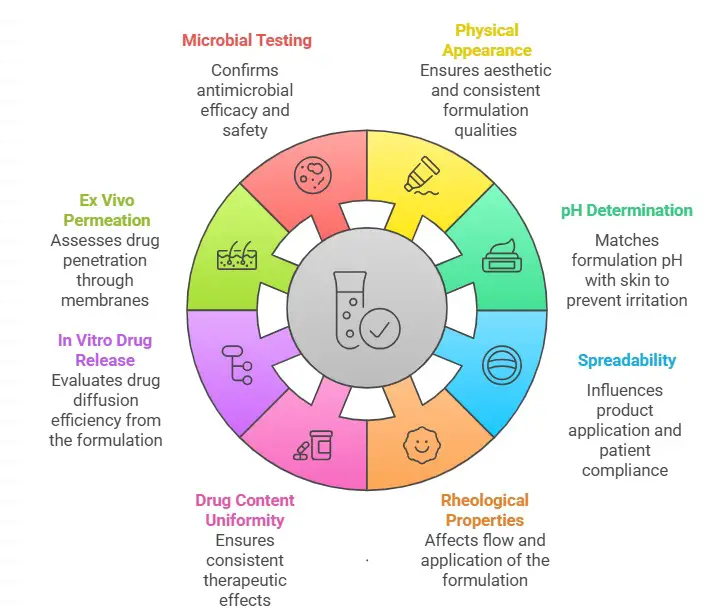
1. Physical Appearance and Organoleptic Properties
The color, odor, and texture of a semi-solid formulation must be consistent and aesthetically acceptable. Physical defects such as phase separation, grittiness, or uneven texture indicate formulation instability.
2. pH Determination
The pH of a semi-solid formulation must match skin’s pH (4.5–6.5) to prevent irritation and ensure stability. pH is measured using a pH meter by dispersing the formulation in distilled water.
3. Spreadability
Spreadability evaluates how easily the product spreads on the skin, influencing patient compliance and drug absorption. The measurement is conducted by placing a fixed amount of the formulation between two glass slides and recording the weight required to spread it to a specific diameter.
4. Rheological Properties (Viscosity and Flow Behavior)
Viscosity defines the flow and consistency of semi-solid formulations, affecting both application and drug release. It is measured using a Brookfield viscometer at different shear rates, and semi-solid formulations should demonstrate shear-thinning (pseudoplastic) behavior for smooth application.
5. Drug Content Uniformity
Ensuring the uniform distribution of the active pharmaceutical ingredient (API) is essential for consistent therapeutic effects. This is achieved by dissolving the sample in a suitable solvent and determining the drug concentration using UV-visible spectrophotometry or high-performance liquid chromatography (HPLC).
6. In Vitro Drug Release Studies
Drug release testing evaluates how efficiently the drug diffuses from the formulation.
Methods:
- Franz Diffusion Cell: A semipermeable membrane separates the donor (formulation) and receptor (buffer solution) compartments. Drug release is measured over time.
- Dissolution Testing: Simulated skin conditions are used to study drug release kinetics.
7. Ex Vivo Permeation Studies
Permeation studies assess drug penetration through biological membranes.
Method:
- Animal skin models (rat, pig skin) or synthetic membranes are used with a Franz diffusion cell.
- The amount of drug diffused is measured via HPLC or UV spectrophotometry.
8. Microbial Testing and Preservative Efficacy
Semi-solid formulations, especially water-containing emulsions, require antimicrobial testing.
Methods:
- Total Microbial Count (TMC) and Pathogen Testing: Confirms compliance with pharmacopeial microbial limits.
- Preservative Challenge Test: Evaluates preservative effectiveness by inoculating the formulation with microbes and monitoring microbial growth over time.
9. Stability Studies
Stability testing determines shelf-life, potency, and physical integrity under different conditions.
Types of Stability Testing:
- Short-Term Stability: Stored at room temperature (25°C, 60% RH).
- Accelerated Stability: Stored at 40°C, 75% RH to predict long-term stability.
- Freeze-Thaw Stability: Evaluates temperature fluctuation effects by cycling between freezing and room temperature.
10. Extrudability (Tube Test)
This test measures how easily a formulation is expelled from a tube or container.
Method:
- A fixed amount of formulation is placed in a collapsible tube, and the required force to extrude it is recorded.
11. Bioadhesion and Mucoadhesion Testing
For formulations requiring prolonged contact with skin or mucosa, bioadhesion properties are assessed.
Method:
- Texture analyzers measure the adhesion force of the formulation to biological membranes.
12. Phase Separation and Syneresis
Phase separation occurs when the oil and water phases of an emulsion-based formulation separate, affecting stability.
Method: Samples are stored under different conditions and observed for liquid exudation or phase separation.
Importance of Evaluating Semi-Solid Dosage Forms
- Ensures uniform drug delivery and therapeutic efficacy.
- Prevents microbial contamination and degradation.
- Enhances patient compliance through texture, spreadability, and absorption.
- Confirm compliance with pharmacopeial and regulatory standards (USP, BP, EP).
Future Trends in Semi-Solid Dosage Forms
1. Nanotechnology-Based Drug Delivery
Nanotechnology enhances drug penetration, stability, and targeted delivery in semi-solid formulations. Liposomes, Nanoemulsions, and nanoparticles improve bioavailability and controlled release, optimizing therapeutic outcomes.
2. Bioadhesive Polymers for Enhanced Retention
Bioadhesive polymers like Carbopol, chitosan, and hyaluronic acid improve skin and mucosal adhesion, prolonging drug action and boosting patient compliance.
3. Advanced Rheological Testing for Stability
Cutting-edge rheological analysis ensures optimal viscosity, spreadability, and phase stability. Techniques like oscillatory rhinometry and shear-thinning analysis enhance product performance.
4. Natural & Biodegradable Excipients
The shift toward eco-friendly excipients like shea butter, xanthan gum, and alginate ensures biocompatibility, sustainability, and reduced toxicity, making formulations safer and greener.
Innovations in nanotechnology, bio adhesion, rheology, and natural excipients are transforming semi-solid drug formulations. These advancements enhance drug delivery, stability, and sustainability, shaping the future of pharmaceutical development.
Conclusion
Evaluating semi-solid dosage forms is essential for quality control, stability assurance, and regulatory compliance. Advanced techniques such as rheological analysis, in vitro permeation studies, and microbial testing ensure that formulations are effective, stable, and safe for patient use. With advancements in nanotechnology, bioadhesive polymers, and novel drug carriers, the future of semi-solid dosage forms will continue to evolve, improving therapeutic efficiency and patient convenience.
Frequently Asked Questions (FAQs)
1. Why is spreadability important in semi-solid formulations?
Answer: Spreadability determines how easily the product can be applied and evenly distributed on the skin, influencing drug absorption and patient compliance.
2. What is the purpose of rheological testing in semi-solid dosage forms?
Answer: Rheological testing assesses viscosity, flow behavior, and texture, ensuring ease of application and consistent drug release.
3. How is in vitro drug release tested for semi-solid formulations?
Answer: In vitro drug release is commonly tested using a Franz diffusion cell, which measures drug diffusion through a membrane over time.
4. What stability tests are performed on semi-solid formulations?
Answer: Stability tests include accelerated stability, freeze-thaw testing, and microbial stability, ensuring long-term effectiveness and shelf-life.
5. How is microbial contamination prevented in semi-solid formulations?
Answer: Microbial contamination is prevented by using preservatives, maintaining aseptic manufacturing conditions, and performing microbial limit tests.