Introduction:
The cyclic structure of benzene, with alternating single and double bonds, has been a subject of considerable scientific interest. Several lines of evidence support the cyclic, rather than the classical Kekulé structures with fixed single and double bonds, for the benzene molecule. Here are some key pieces of evidence:
(1) Substitution of Benzene:
Benzene reacted with bromine in the presence of FeBr3(catalyst) to form monobromobenzene.

The fact that only one monobromo and no isomeric products were obtained indicated that all six hydrogen atoms in benzene were identical. This could be possible only if benzene had a cyclic structure of six carbons and one hydrogen was attached to each carbon.
2. Addition of Hydrogen:
Benzene exhibits unusual behavior in hydrogenation reactions. According to classical structures, adding three moles of hydrogen should yield cyclohexane. However, experimental observations show that only one mole of hydrogen adds to benzene, indicating equal bond strengths and a lack of distinct single and double bonds.
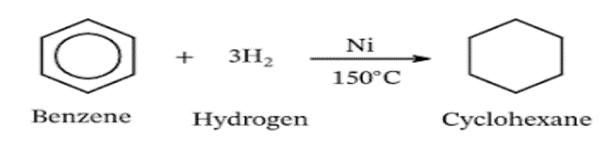
3. Isomer Counting:
According to classical structures, there should be three isomers of dichlorobenzene. However, only one isomer is observed, supporting the idea that all carbon-carbon bonds in benzene are equivalent.
4. Molecular Geometry:
X-ray crystallography and other techniques reveal that all carbon-carbon bond lengths in benzene are equal. This is inconsistent with the alternating single and double bond structure but supports a more symmetric, delocalized electron distribution.
5. Heat of Hydrogenation:
The heat of hydrogenation for benzene is less than expected based on classical structures. If benzene had alternating single and double bonds, it would be expected to have higher reactivity, leading to a higher heat of hydrogenation.
6. Magnetic Properties:
Benzene is diamagnetic, meaning it is not attracted to a magnetic field. According to classical structures, benzene would be expected to be attracted, but the observed lack of attraction is consistent with a delocalized electron cloud.
7. Resonance Theory:
The concept of resonance was introduced to explain benzene’s structure. Resonance allows for representing multiple structures (with alternating single and double bonds) without specifying a fixed arrangement, indicating the delocalization of electrons.
8. Spectroscopy:
Spectroscopic techniques, such as NMR (nuclear magnetic resonance), support the idea of electron delocalization in benzene. The signals observed in NMR are consistent with a structure where the electrons are spread out over all six carbon atoms.
This evidence collectively supports the understanding that benzene has a more stable and unique cyclic structure with delocalized electrons, challenging the classical Kekulé structures. The resonance hybrid model provides a more accurate representation of the actual electron distribution in the benzene molecule.