Meta Description:
Discover the preparation, benefits, and medicinal uses of Ferrous Sulphate (FeSO₄). Learn how it treats anemia, supports pregnancy, and its role in industry. Plus, key side effects & precautions!
Introduction
Ferrous sulphate (FeSO₄) is a widely used iron supplement with significant roles in medicine, industry, and agriculture. It is primarily known for its effectiveness in treating iron deficiency anemia, a common condition affecting millions worldwide. Beyond healthcare, ferrous sulphate is essential in water purification, fertilizer production, and industrial applications.
This article provides a comprehensive guide on ferrous sulphate, covering its preparation methods, assay techniques, key properties, and medicinal benefits. Whether you’re a healthcare professional, researcher, or simply looking to understand how ferrous sulphate works, this guide will give you valuable insights into its applications, benefits, and potential side effects.
General Methods of Preparation of Ferrous Sulphate
Ferrous sulphate can be synthesized through several methods, primarily involving iron and sulfuric acid reactions. Below are the most common preparation techniques:
1. Reaction of Iron with Sulfuric Acid
This is the most common industrial method.
Chemical Reaction:
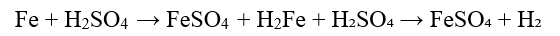
Process:
- – Iron filings or scrap metal are dissolved in dilute sulfuric acid.
- – The reaction produces ferrous sulphate and hydrogen gas.
- – The solution is then filtered and crystallized to obtain ferrous sulphate heptahydrate (FeSO₄·7H₂O).
2. Oxidation of Pyrite (FeS₂) or Siderite (FeCO₃)
Chemical Reactions:
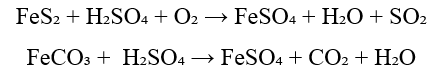
Process:
- – Pyrite or siderite ore is treated with dilute sulfuric acid.
- – The reaction yields ferrous sulphate and additional by-products.
3. Byproduct from Steel Manufacturing
- – Ferrous sulphate is often recovered as a byproduct of steel pickling using sulfuric acid.
- – The waste acid is processed and crystallized to extract ferrous sulphate.
Assay of Ferrous Sulphate
The assay of ferrous sulphate is conducted using a redox titration method with potassium permanganate (KMnO₄).
Procedure:
1. Dissolve a weighed sample of ferrous sulphate in dilute sulfuric acid.
2. Titrate with standard KMnO₄ solution until a persistent pink color appears.
The reaction follows:
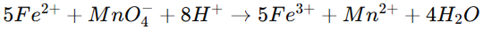
The volume of KMnO₄ used determines the purity of ferrous sulphate.
Properties of Ferrous Sulphate
Chemical Properties:
Molecular Formula: FeSO₄·xH₂O (commonly FeSO₄·7H₂O)
Molecular Weight: 278.0 g/mol (heptahydrate)
Solubility: Soluble in water, insoluble in alcohol
pH: Slightly acidic in aqueous solution
Oxidation: Slowly oxidizes in air to form ferric sulphate (Fe₂(SO₄)₃)
Physical Properties:
Appearance: Pale green or bluish-green crystalline solid
Taste: Astringent and metallic
Stability: Moisture-sensitive; decomposes upon exposure to air
Medicinal Uses of Ferrous Sulphate
Ferrous sulphate is widely used in medicine for its iron-supplying properties. The key applications include:
1. Treatment of Iron Deficiency Anemia: Ferrous sulphate provides essential iron for hemoglobin synthesis. Typically, 200-300 mg per day in divided doses. Taken orally with vitamin C to enhance absorption.
2. Prenatal Supplementation: Prescribed to pregnant women to prevent iron deficiency and support fetal development.
Often combined with folic acid for better effectiveness.
3. Chronic Kidney Disease (CKD) Management: Used as an iron supplement in CKD patients receiving erythropoietin therapy.
4. Industrial and Other Applications
- Water Treatment: Used to remove contaminants in wastewater.
- Agriculture: Acts as a fertilizer and pesticide to correct iron deficiency in plants.
Side Effects and Precautions Ferrous Sulphate
While ferrous sulphate is generally safe, excessive intake may cause side effects, including:
1. Gastrointestinal Issues
- – Common Effects: Nausea, constipation, diarrhea
- – Prevention: Take with food or in a slow-release formulation.
2. Iron Overload (Hemochromatosis)
- – Symptoms: Liver damage, joint pain, heart problems
- – Prevention: Avoid unnecessary iron supplementation.
3. Drug Interactions
- – Avoid with: antacids, tetracyclines, and calcium supplements (reduce absorption).
- – Enhancers: Vitamin C improves iron absorption.
Conclusion
Ferrous sulphate is an important iron supplement used in anemia treatment, pregnancy support, and industrial applications. Its preparation methods, assays, properties, and medicinal benefits make it a crucial compound in medicine and industry. However, proper dosage and precautions must be followed to prevent side effects.
By understanding its functions and proper usage, individuals can ensure effective iron supplementation and overall health improvement.
Frequently Asked Questions (FAQs)
1. What is ferrous sulphate used for?
Answer: It is primarily used for iron deficiency anemia, prenatal care, and CKD treatment.
2. How should ferrous sulphate be taken?
Answer: It should be taken on an empty stomach for better absorption, but with food if gastric discomfort occurs.
3. Can ferrous sulphate be taken daily?
Answer: Yes, but only as per medical advice to avoid iron overload.
4. What is the difference between ferrous sulphate and ferric sulphate?
Answer: Ferrous sulphate (FeSO₄) is more bioavailable and commonly used in supplements.
Ferric sulphate (Fe₂(SO₄)₃) is less soluble and mainly used in industrial applications.
5. What are natural sources of iron?
Answer: Plant sources: Spinach, lentils, fortified cereals reduce iron absorption. Instead, pair it with vitamin C-rich foods for better results.
Animal sources: Red meat, liver, fish