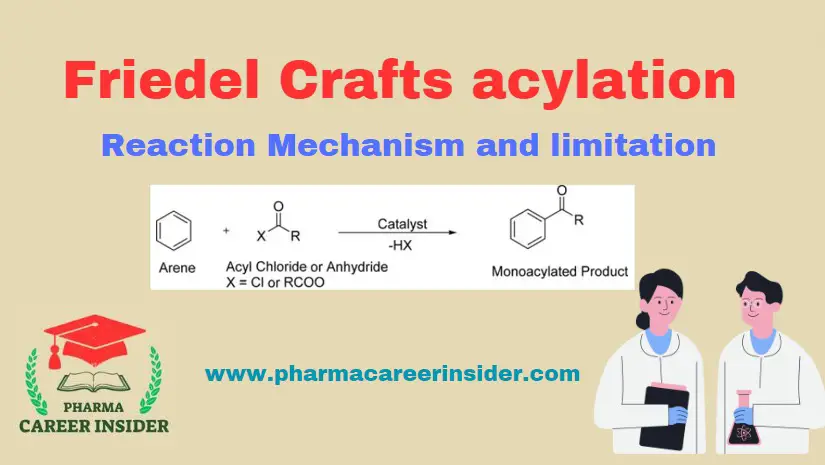
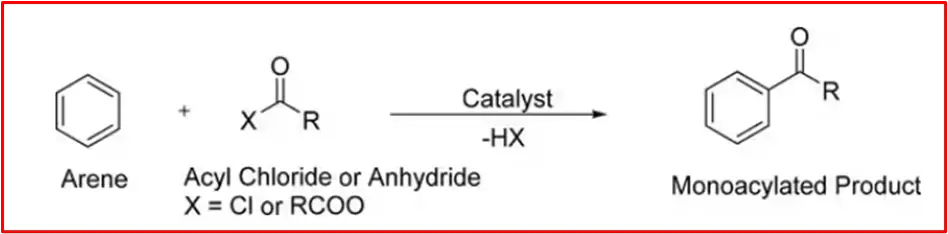
The Friedel-Crafts acylation reaction involves adding an acyl group to an aromatic ring. Typically, this is done by employing an acid chloride (R-(C=O)-Cl) and a Lewis acid catalyst such as AlCl3. The aromatic ring is transformed into a ketone in a Friedel-Crafts acylation reaction. The reaction between benzene and acyl chloride under these conditions is illustrated below.
Mechanism:
Step 1:
In this step, the acyl chloride (R−C(O)−Cl) reacts with the Lewis acid catalyst (AlCl3) to form the acylium ion (R−C(O)+) and an aluminium chloride complex (AlCl4−).
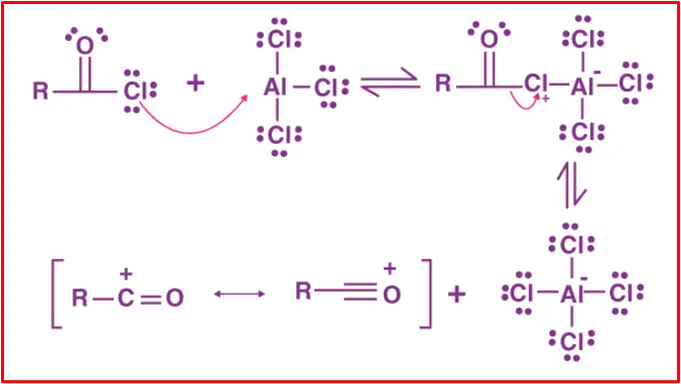
Step 2:
The acylium ion (R−C(O)+) acts as an electrophile, attacking the aromatic ring (Ar−H). This forms a sigma complex, where the aromatic ring undergoes temporary sp³ hybridization.
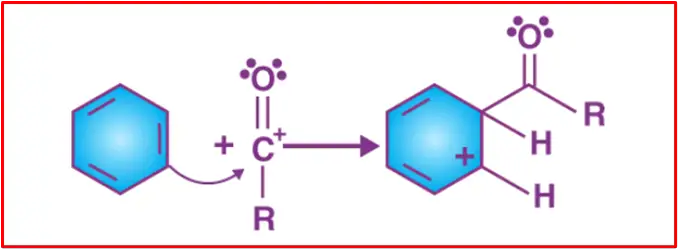
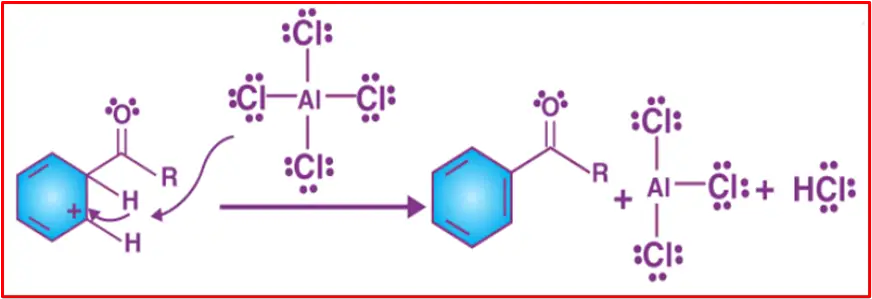
Step 3:
The intermediate complex undergoes deprotonation, bringing back the aromaticity to the ring. The proton, released in this process, combines with a chloride ion (originating from the Lewis acid complex) to create HCl. This results in the regeneration of the AlCl₃ catalyst.
Limitations of Friedel-Crafts Acylation:
- Carbocation Rearrangement: Like Friedel-Crafts alkylation, carbocation rearrangement can occur during acylation. This is more significant with secondary and tertiary alkylating agents, leading to undesired products.
- Polyacylation: Aromatic compounds can undergo multiple acylation reactions, resulting in polyacylated products. Controlling the reaction to achieve monoacylation can be challenging.
- Direct Alkylation Preferred: In many cases, Friedel-Crafts alkylation is preferred over acylation due to its simplicity and higher yield of monoalkylated products.
- Substrate Limitations: Some substrates may not react well in Friedel-Crafts acylation reactions, especially those with strong electron-withdrawing groups.
- Catalyst Reactivity: The Lewis acid catalysts used, such as AlCl₃, can react with certain functional groups present in the substrate, leading to side reactions and decreased yield.
- Aromatic Substrate Requirement: Friedel-Crafts acylation is specific to aromatic substrates and does not apply to aliphatic compounds.
- Steric Hindrance: Bulky substituents on the aromatic ring or the acylating agent can hinder the reaction, affecting regioselectivity and yield.
- Side Reactions: Unwanted side reactions, such as over-acylation or polymerization, can occur under certain conditions, leading to complex mixtures.
- Temperature Sensitivity: Friedel-Crafts acylation can be sensitive to reaction temperatures, and elevated temperatures may favour side reactions or catalyst decomposition.