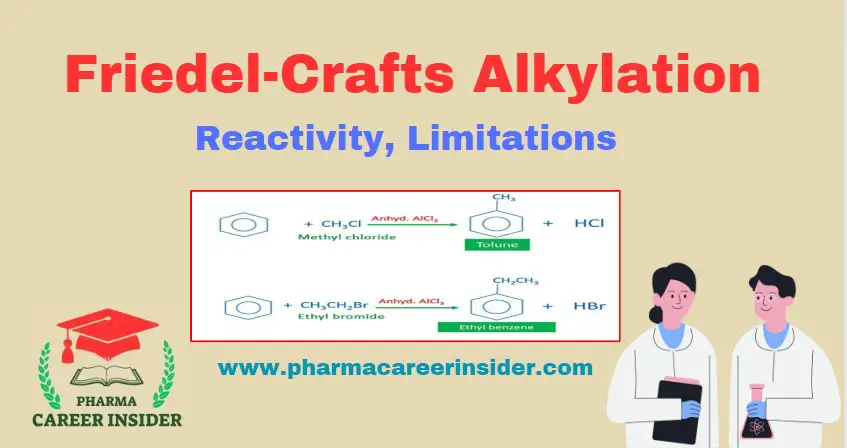
Reactivity:
Friedel-Crafts alkylation is a powerful method for introducing alkyl groups onto aromatic rings. The reaction involves the electrophilic substitution of a hydrogen atom on the aromatic ring with an alkyl group. The key steps include the generation of an alkyl cation as the electrophile and its subsequent attack on the aromatic ring. Lewis’s acid catalysts, such as aluminium chloride (AlCl₃) or ferric chloride (FeCl₃), play a crucial role in facilitating this process.
Benzene reacts with alkyl halides in the presence of aluminium chloride to form alkylbenzenes. For example,
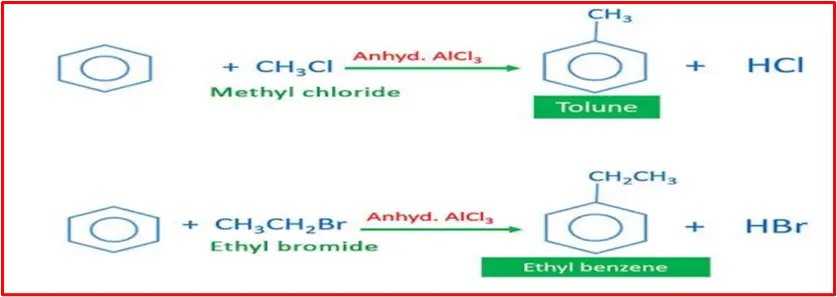
Mechanism:
The following steps are involved:
Step 1: Formation of the electrophile (CH3+)
In this step, the alkyl halide (e.g., CH3Cl) reacts with the Lewis acid catalyst (e.g., AlCl3) to generate the electrophile, a methyl cation (CH3+), along with the formation of an aluminium chloride complex.
Step 2: The electrophile attacks the benzene ring to give a carbonium ion
The generated methyl cation (CH3+) acts as an electrophile, attacking the benzene ring. This leads to forming a sigma complex (carbonium ion), where one carbon in the benzene ring becomes temporarily sp³ hybridized.
Step 3: loss of proton gives alkylbenzene.
The sigma complex undergoes a rearrangement, and a proton is lost, forming the final product, alkyl benzene.
Limitations and Considerations:
Carbocation Rearrangement:
Friedel-Crafts alkylation can lead to carbocation rearrangements, especially when using secondary or tertiary alkylating agents. This can result in undesired products.
Polyalkylation:
Aromatic compounds can undergo multiple alkylation reactions, leading to polyalkylated products. This complicates the reaction and makes it challenging to control the regioselectivity.
Friedel-Crafts Acylation Preference:
Friedel-Crafts acylation is often preferred over alkylation due to its higher regioselectivity and avoidance of carbocation rearrangements.
Side Reactions:
Friedel-Crafts alkylation is prone to side reactions such as polymerization and Friedel-Crafts reactions with the alkylating agent.
Catalyst Handling:
The corrosive nature of the Lewis acid catalysts used in the reaction requires careful handling and can lead to catalyst contamination in the product.