Half-Life
Meta Description
Understand half-life: definition, formula, and real-world applications in nuclear physics, medicine, and archaeology. Learn how radioactive decay impacts science.
Introduction
Half-life is a fundamental concept innuclear physics, chemistry, and medicine, referring to the time required for half of a radioactive substance to decay. Understanding half-life is crucial in fields such as radiopharmaceuticals, radiocarbon dating, nuclear energy, and radiation safety.
This article provides explanation of half-life, including its formula, calculation, factors affecting decay, and real-world applications.
What is half-life?
The half-life (t₁/₂) of a substance is the time it takes for half of its atoms to undergo radioactive decay. It is a constant property for a given isotope and helps in predicting how long a radioactive material remains active.
For example, the half-life of carbon-14 is about 5,730 years, meaning after this time, only 50% of the original carbon-14 remains.
Half-Life Formula
The mathematical equation for half-life follows the first-order decay law:
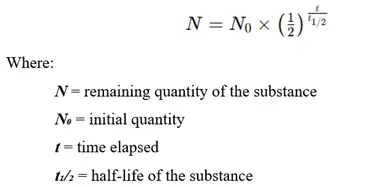
Alternatively, the relationship between half-life and the decay constant (λ) is:
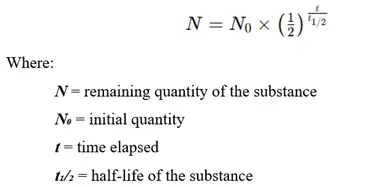
This equation helps in calculating the decay rate and understanding radioactive transformations.
How is half-life calculated?
Example Calculation
1. Problem: If a 100g sample of iodine-131 (half-life = 8 days) is left for 24 days, how much remains?
Solution:
Number of half-lives passed = 24 / 8 = 3
Remaining mass = 100 × (1/2)³ = 100 × 1/8 = 12.5g
Thus, after 24 days, only 12.5g of iodine-131 remains.
2. Problem: A wooden artifact contains 80g of carbon-14. Given that carbon-14 has a half-life of 5,730 years, how much carbon-14 will remain after 11,460 years?
Solution:
Number of half-lives passed = 11,460 / 5,730 = 2
Remaining mass = 80 × (1/2)² = 80 × 1/4 = 20g
Answer: After 11,460 years, only 20 g of carbon-14 remains in the artifact.
Factors Affecting Half-Life
- 1. Isotope Properties: Each element has a unique half-life, depending on nuclear stability.
- 2. Environmental Conditions: Temperature, pressure, and chemical interactions do not alter half-life.
- 3. External Radiation Exposure: High-energy interactions can sometimes influence nuclear decay rates.
Applications of Half-Life
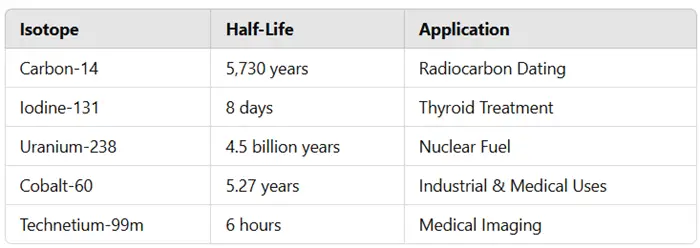
1. Radiocarbon Dating (C-14 Dating)
- Used to determine the age of ancient fossils and artifacts.
- Carbon-14 decays with a half-life of 5,730 years, making it ideal for dating organic materials up to 50,000 years old.
2. Medical Applications (Radiopharmaceuticals)
- Technetium-99m (t₁/₂ = 6 hours) is widely used in diagnostic imaging.
- Iodine-131 (t₁/₂ = 8 days) is used in thyroid cancer treatment.
3. Nuclear Power and Waste Management
- Uranium-235 (t₁/₂ = 704 million years) is used in nuclear reactors.
- Understanding half-life helps in radioactive waste disposal and safety measures.
4. Radiation Therapy
- Half-life is crucial in selecting isotopes for cancer radiation therapy.
5. Security and Industrial Applications
- Cobalt-60 (t₁/₂ = 5.27 years) is used in industrial radiography and sterilization of medical equipment.
Comparison of Common Isotopes and Their Half-Lives
Conclusion
Half-life is a vital concept in physics, chemistry, and medicine, affecting everything from nuclear power generation to medical diagnostics and archaeological dating. Understanding the half-life of elements helps in safety planning, isotope selection, and effective scientific applications.
By mastering half-life calculations and real-world applications, scientists and researchers can make informed decisions in nuclear energy, medicine, and environmental science.
Frequently Asked Questions (FAQs)
1. What does half-life mean in radioactive decay?
Answer: Half-life is the time required for half of a radioactive substance to decay, reducing its mass by 50%.
2. How is half-life calculated?
Answer: Half-life is calculated using the formula t₁/₂ = 0.693 / λ, where λ is the decay constant. The remaining quantity can be determined by N = N₀ (1/2)(t/t₁/₂).
3. Why is half-life important in nuclear medicine?
Answer: Medical isotopes like Technetium-99m (t₁/₂ = 6 hours) help in imaging, while Iodine-131 (t₁/₂ = 8 days) is used in thyroid treatment.
4. What factors affect the half-life of an element?
Answer: Half-life is a constant property of a radioactive isotope and is not affected by temperature, pressure, or chemical changes.
5. What are common applications of half-life?
Answer: Half-life is crucial in radiocarbon dating, cancer therapy, nuclear power generation, and industrial sterilization.
Have questions about radioactive decay? Drop a comment below or share this article!