1. Introduction to Iodimetry:
Iodimetry is a type of volumetric analysis that involves the use of iodine (I₂) or iodine-containing compounds as titrants to determine the concentration of reducing agents in a solution. The key reaction in iodimetry is the reduction of iodine (I₂) to iodide ions (I⁻) by a reducing agent. Iodimetry is commonly used for determining the concentration of substances such as ascorbic acid, sulfur dioxide, and various metal ions that act as reducing agents.
Iodimetry can be classified as either direct iodimetry, where iodine solution is directly used as the titrant, or indirect iodimetry, where iodine is generated in situ by an oxidizing agent and then titrated.
2. Principle of Iodimetry:
The principle behind iodimetry is based on the redox reaction between iodine (I₂) and a reducing agent in the sample. Iodine is reduced to iodide (I⁻), and the reducing agent is oxidized in the process.
Reduction of iodine (I₂):
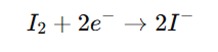
The sample contains a substance that is a reducing agent (such as ascorbic acid, sulfur dioxide, or iron (II) ions), which donates electrons to reduce iodine (I₂) to iodide (I⁻).
The endpoint of the titration is determined by detecting when all the reducing agents in the sample have reacted with iodine.
3. Procedure of Iodimetry:
The following steps outline the typical procedure for performing iodimetric titration:
Preparation of the sample:
- Prepare the sample solution that contains the reducing agent to be analyzed. The sample must be free from oxidizing agents that could interfere with the titration.
- For substances like sulfur dioxide or ascorbic acid, the sample is usually dissolved in water or an acidic medium.
Preparation of iodine solution:
- A standard iodine solution (I₂) is used as the titrant. This solution is typically prepared by dissolving iodine crystals in a potassium iodide (KI) solution, as iodine is more soluble in iodide ions.
- The iodine solution is often standardized using a primary standard, such as sodium thiosulfate.
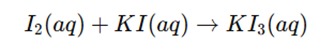
Titration:
- The iodine solution is added from a burette to the sample containing the reducing agent.
- During the titration, the iodine (I₂) is reduced to iodide ions (I⁻) by the reducing agent present in the sample.
Detection of the endpoint:
- The endpoint is detected by the addition of a starch indicator. Starch forms a blue complex with iodine, and the endpoint is reached when the blue color no longer appears, indicating that all the iodine has reacted with the reducing agent.
- If starch is not used, the appearance of a faint yellow or pale color can indicate the endpoint, as iodine becomes free in the solution when reducing agents are consumed.
Calculation:
The concentration of the reducing agent in the sample can be calculated using the volume of iodine solution required for the titration and its concentration.
The amount of iodine used in the reaction corresponds to the amount of reducing agent present in the sample. The calculation follows the equation:
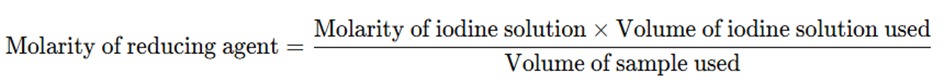
4. Applications of Iodimetry:
Iodimetry is widely used for determining reducing agents in various fields. Some key applications include:
1. Analysis of Ascorbic Acid (Vitamin C):
Iodimetry is commonly used to quantify ascorbic acid in food, pharmaceuticals, and biological samples because ascorbic acid acts as a reducing agent that can reduce iodine to iodide.
2. Determination of Sulfur Dioxide:
Iodimetry is used to determine the concentration of sulfur dioxide (SO₂) in air, food, and beverages. Sulfur dioxide is a reducing agent that reduces iodine to iodide ions.
3. Analysis of Iron (II) (Fe²⁺) ions:
Iron (II) ions are frequently determined using iodimetry by reducing iodine to iodide. Iron (III) (Fe³⁺) is reduced to iron (II) in the presence of a reducing agent, and iodine is titrated against the sample.
4. Determination of Copper (I) and Copper (II):
Copper (I) and copper (II) can be quantified using iodimetric titration, as copper (I) acts as a reducing agent that can reduce iodine to iodide ions.
5. Pharmaceutical Quality Control:
Iodimetry is used in the quality control of drugs, such as in the determination of antioxidant content or in the evaluation of the effectiveness of certain formulations.
6. Analysis of Peroxides:
Iodimetry is employed to determine the concentration of peroxides (such as hydrogen peroxide) in solutions, as peroxides act as reducing agents capable of reducing iodine.
5. Advantages and limitations:
- Advantages:
- Simple and reliable: Iodimetry is easy to perform and provides reliable results with minimal equipment.
- Rapid and cost-effective: The process requires a relatively short time and is inexpensive compared to other methods.
- Widely applicable: It can be used for a broad range of reducing agents.
- High sensitivity: Iodimetry is particularly sensitive for the detection of reducing agents in low concentrations.
- Limitations:
- Interference from oxidizing agents: The presence of oxidizing agents in the sample can interfere with the titration process and lead to inaccurate results.
- Stability of iodine solution: Iodine solutions are sensitive to light and air and may degrade over time, requiring careful storage and handling.
- Use of starch indicator: Starch must be carefully used as an indicator, as it can give a false color reaction if added in excess or too early in the titration.
In summary, iodimetry is an effective redox titration method used for determining the concentration of reducing agents in various samples. With applications in pharmaceutical, environmental, and food analysis, iodimetry remains a widely used technique in analytical chemistry.


