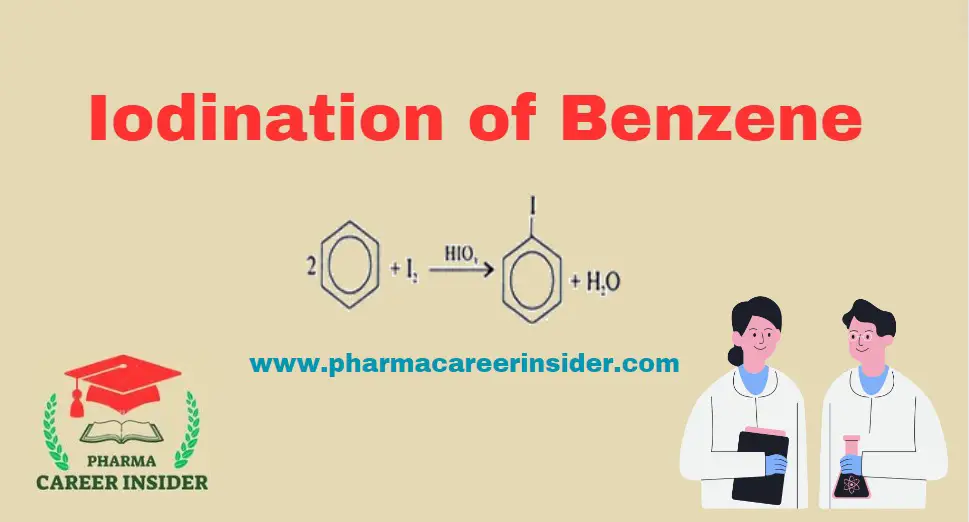
The iodination of benzene involves the substitution of a hydrogen atom on the benzene ring with an iodine atom through an electrophilic aromatic substitution (EAS) reaction. Here is the reaction with a concise explanation:
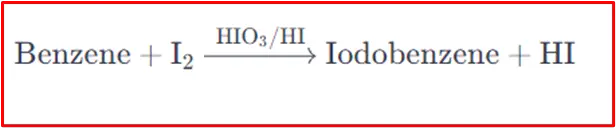
Here is a step-by-step mechanism for the iodination of benzene:
Step 1: Formation of electrophile
Iodine reacts with iodic acid (HIO3)to form an iodonium ion (I+), an iodate ion (IO3-) and a proton (H+). The iodonium ion is the electrophile responsible for attacking the benzene ring.
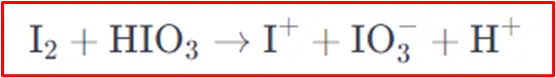
Step 2: Formation of carbocation The iodonium ion (I+) attacks the benzene ring, forming a sigma complex.

Step 3: Protonation A proton is lost from the sigma complex, forming a stabilized aromatic intermediate with an iodine substituent.