1. Introduction to Iodometry
Iodometry is a type of volumetric analysis that involves the determination of an oxidizing agent by titrating it with a standard solution of sodium thiosulfate (Na₂S₂O₃). In an iodometric titration, iodine (I₂) is generated in situ by the reaction between an oxidizing agent and excess iodide ions. The iodine is then titrated with a reducing agent, typically sodium thiosulfate until the endpoint is reached.
Iodometry is widely used to determine the concentration of oxidizing agents in a variety of samples, including chlorine, hydrogen peroxide, and other substances with strong oxidizing properties.
2. Principle of Iodometry
The principle of iodometry is based on the oxidation-reduction reaction, where an oxidizing agent in the sample oxidizes iodide ions (I⁻) to iodine (I₂). The iodine (I₂) generated is then titrated with sodium thiosulfate, a reducing agent, which reduces iodine back to iodide ions (I⁻).
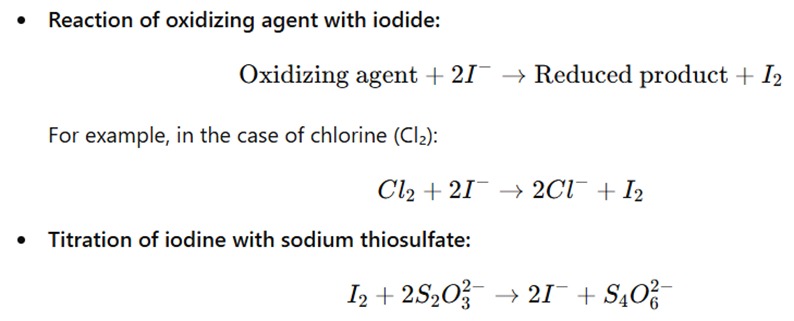
The amount of iodine generated is directly proportional to the amount of oxidizing agent in the sample, and the iodine is quantified by titrating with a standard solution of sodium thiosulfate.
3. Procedure of Iodometry
The typical steps in performing an iodometric titration are as follows:
(a) preparation of the sample:
- Dissolve the sample containing the oxidizing agent in a suitable solvent (usually water). If necessary, adjust the pH to make the solution slightly acidic to facilitate the reaction.
- Ensure that the sample is free from any interfering substances, especially reducing agents.
(b) Addition of excess iodide:
- Add an excess amount of potassium iodide (KI) to the sample solution. The iodide ions (I⁻) react with the oxidizing agent in the sample to liberate iodine (I₂).
- The iodine formed is typically in the form of a brownish-yellow solution.
(c) Preparation of sodium thiosulfate solution:
- A standard solution of sodium thiosulfate (Na₂S₂O₃) is used as the titrant. This solution is typically prepared by dissolving a known amount of sodium thiosulfate in distilled water.
- The sodium thiosulfate solution is standardized using a primary standard, such as potassium dichromate.
(d) Titration:
- Fill a burette with the sodium thiosulfate solution.
- Titrate the iodine-containing solution with sodium thiosulfate, adding it dropwise while stirring continuously.
- As the iodine is reduced to iodide by sodium thiosulfate, the brown color of iodine gradually fades. Continue titrating until the solution becomes colorless.
(e) Use of starch indicator (optional):
Starch solution is often used as an indicator in iodometry. When iodine is present in solution, the starch forms a blue complex with iodine. As the iodine is reduced to iodide, the blue color disappears, indicating the endpoint of the titration.
(f) Calculation:
The concentration of the oxidizing agent in the sample can be calculated based on the volume of sodium thiosulfate solution used for titration. The reaction between iodine and sodium thiosulfate is stoichiometric, so the amount of iodine generated corresponds to the amount of oxidizing agent in the sample.
The equation for the calculation is:
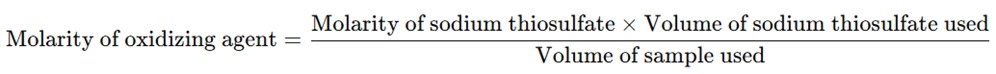
4. Applications of Iodometry
Iodometry is widely used in chemical analysis to quantify oxidizing agents in a variety of applications. Some key applications include:
(a) Determination of chlorine: Iodometry is commonly used to determine the concentration of chlorine gas (Cl₂) or chlorine compounds, such as sodium hypochlorite (NaOCl) and chlorine water. Chlorine oxidizes iodide ions to iodine, and the iodine is then titrated with sodium thiosulfate.
(b) Analysis of Hydrogen Peroxide (H₂O₂): Iodometry is used to determine the concentration of hydrogen peroxide, as hydrogen peroxide is a strong oxidizing agent that can liberate iodine from iodide ions.
(c) Analysis of Copper (II) Compounds: Copper (II) compounds (such as copper sulfate, CuSO₄) can be analyzed using iodometry, where copper (II) is reduced by iodide ions, generating iodine that is then titrated.
(d) Analysis of Oxidizing Agents in Water: Iodometry is used in water treatment and environmental analysis to determine the concentration of oxidizing agents such as ozone (O₃) and chlorine in water samples.
(e) Determination of Hypochlorite in Bleaching Agents: Sodium hypochlorite in bleaching agents is commonly analyzed by iodometry. The hypochlorite liberates iodine, which is titrated with sodium thiosulfate.
(f) Determination of Copper (I) in Solutions: Iodometry is also used to determine copper (I) ions in solutions, as copper (I) is oxidized by iodine to form copper (II).
5. Advantages and Limitations
(a) Advantages:
- High precision and accuracy: Iodometry is a reliable and widely accepted method for determining the concentration of oxidizing agents.
- Simple procedure: The method is straightforward and does not require complex equipment.
- Wide applicability: It can be used to analyze a wide variety of samples containing oxidizing agents.
(b) Limitations:
- Interference from reducing agents: The presence of reducing agents other than iodide ions can interfere with the reaction and affect the accuracy of the results.
- Sensitivity to light: Iodine is sensitive to light and can undergo photodecomposition, which may affect the accuracy if the titration is performed under improper conditions.
- Standardization requirement: The sodium thiosulfate solution needs to be carefully standardized before use, as its concentration can change over time due to the absorption of moisture.
In summary, iodometry is an effective and widely used method for determining the concentration of oxidizing agents. By liberating iodine from iodide ions and titrating the iodine with sodium thiosulfate, iodometry allows for precise measurements of oxidizing agents in a variety of chemical, pharmaceutical, and environmental analyses.




Good explanation.