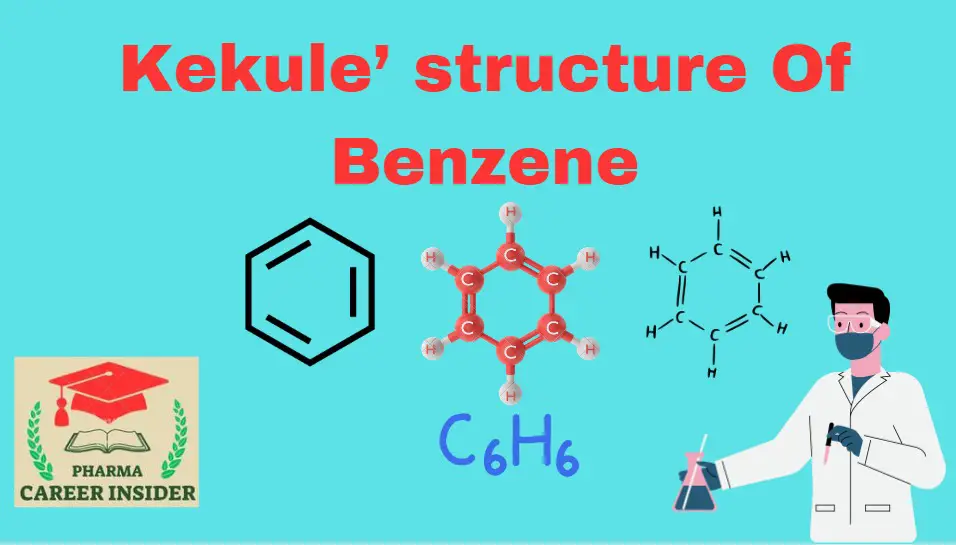
Introduction:
In 1865, Kekule suggested benzene consisted of a cyclic planar structure of six carbons with alternate double and single bonds. To each carbon was attached one hydrogen. Benzene, according to this proposal, was simple 1,3,5-cyclohexatriene.
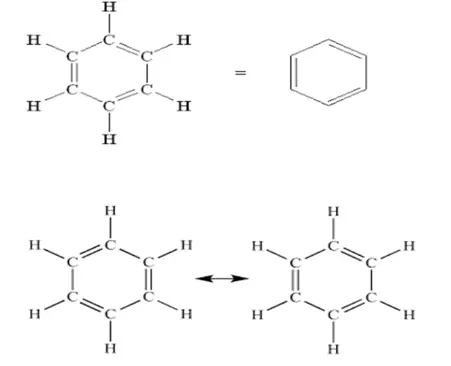
Kekule’s structure of benzene explained satisfactorily the following points.
1. That benzene contains three double bonds.
2. All six hydrogen atoms in benzene are equivalent, and benzene gives only one monosubstituted product, C6H5X.
3. Three possible disubstituted benzene products (ortho, meta, para).
Objections to Kekule’s Structure:
The structure of benzene postulated by Kekule could not explain the following facts:
1. From Kekule’s structure, one should expect that benzene, due to the presence of three double bonds, should show chemical properties similar to alkenes, but it does not do so. Benzene does not decolorize the purple color of alkaline KMnO4 or the orange-red color of bromine water.
2. based on Kekule’s structure, two ortho Dibromobenzene are possible. In one structure, a single bond links the two carbon atoms to which the Br atoms are attached, while in another structure, they are connected by a double bond. In reality, we only know one ortho dibromo benzene.
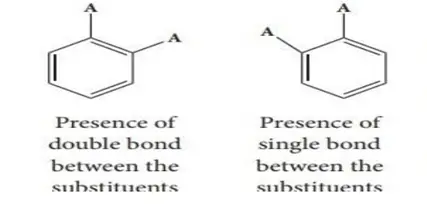
To explain this, Kekule postulated that benzene may be a mixture of two rapidly interconverting forms in which the single and the double bonds rapidly interconvert.
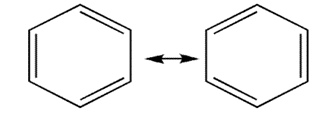
3. The suggestion that the benzene molecule has three alternate double bonds is incorrect.
Conclusion:
Benzene’s actual structure is a resonance hybrid reflecting electrons’ delocalization over all six carbon atoms. The resonance hybrid model provides a more accurate depiction of the stability and properties observed in benzene.