Introduction
The Limit Test for Chlorides is a pharmacopeial test used in pharmaceutical quality control to detect and quantify chloride impurities in drugs, chemicals, and raw materials. Since high chloride levels can cause corrosion, stability issues, and degradation in pharmaceutical formulations, this test ensures that the chloride content remains within the prescribed limits.
Various pharmacopeias, such as the Indian Pharmacopoeia (IP), British Pharmacopoeia (BP), and United States Pharmacopoeia (USP), specify this limit test to maintain drug purity, safety, and efficacy.
Principle of the Limit Test for Chlorides
The test is based on the precipitation reaction between chloride ions (Cl⁻) and silver nitrate (AgNO₃) in the presence of dilute nitric acid (HNO₃). This reaction results in the formation of insoluble silver chloride (AgCl), which appears as a white precipitate.
The intensity of this turbidity in the test solution is compared visually with that of a standard chloride solution containing a known amount of chloride ions.
Chemical Reaction Involved
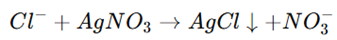
Here,
- AgCl is an insoluble white precipitate, indicating the presence of chloride.
- Nitric acid (HNO₃) prevents interference by dissolving unwanted impurities.
Procedure for the Limit Test for Chlorides
Materials Required
- – Nessler cylinders
- – 0.1 M Silver Nitrate (AgNO₃) solution
- – Dilute Nitric Acid (HNO₃)
- – Standard Chloride Solution (25 ppm Cl⁻)
- – Distilled water
- – Glass rod
Step-by-Step Procedure
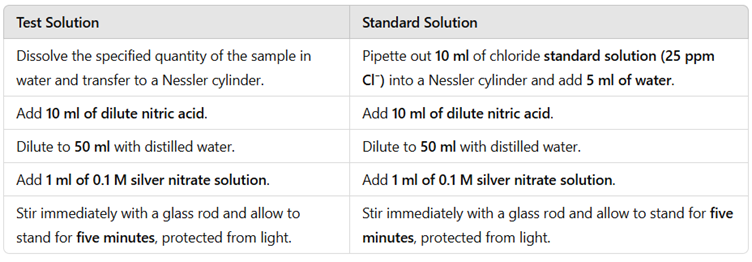
Interpretation of Results
- Pass: If the turbidity in the test solution is less than or equal to the standard solution, the sample meets the chloride limit.
- Fail: If the test solution shows more turbidity, it contains excess chloride and does not comply with pharmacopeial standards.
Significance of the Limit Test for Chlorides
- 1. Ensures Drug Purity: Removes unwanted chloride contaminants.
- 2. Prevents Drug Degradation: Excess chlorides can cause chemical instability.
- 3. Regulatory Compliance: Required by USP, BP, IP for pharmaceutical approval.
- 4. Enhances Drug Safety: Limits toxic effects of excess chloride.
- 5. Quality Control: Helps in maintaining batch-to-batch consistency in pharmaceutical manufacturing.
Common Sources of Chloride Contamination
- Raw materials used in drug formulations
- Water used in pharmaceutical processes
- Manufacturing equipment and pipelines
- Impurities in excipients and solvents
To minimize chloride contamination, manufacturers follow Good Manufacturing Practices (GMP) and use purified water in formulations.
Challenges and Limitations of the Limit Test for Chlorides
Despite its importance, the Limit Test for Chlorides has some limitations:
- Visual method: Since turbidity is assessed visually, small differences may not be detected accurately.
- Interference: Other substances in the solution may affect the reaction.
- Not quantitative: This test only detects the presence of chlorides but does not determine the exact concentration.
For more precise chloride quantification, titrimetric methods or ion chromatography are used.
Comparison with Other Limit Tests

Conclusion
The Limit Test for Chlorides is an essential quality control test in pharmaceuticals, ensuring that chloride impurities remain within acceptable limits. It prevents drug instability, degradation, and safety concerns. Although simple and effective, it has some limitations, and advanced quantitative methods may be required for precise analysis.
By following pharmacopeial guidelines, pharmaceutical manufacturers can maintain high-quality standards and ensure the safety of medicines for consumers.