Introduction
In pharmaceutical analysis, the Limit Test for Iron is an essential quality control test that detects and limits iron impurities in drug substances. Excess iron can cause drug degradation, discoloration, and reduced efficacy. Regulatory bodies like the Indian Pharmacopoeia (IP), British Pharmacopoeia (BP), and United States Pharmacopoeia (USP) mandate strict limits on iron content in pharmaceutical substances to ensure drug safety and stability.
This article explains the principle, procedure, interpretation, and importance of the Limit Test for Iron in pharmaceutical quality control.
What is the Limit Test for Iron?
The Limit Test for Iron is a semi-quantitative test used to detect traces of iron (Fe³⁺ ions) in pharmaceutical substances. The test is based on the reaction between iron (Fe³⁺) and thioglycolic acid in an alkaline medium, forming a pink to reddish-purple color. The intensity of this color is compared with a standard iron solution to determine whether the sample meets the permissible limit.
Principle of the Limit Test for Iron
The test relies on the ability of thioglycolic acid (HSCH₂COOH) to reduce ferric ions (Fe³⁺) to ferrous ions (Fe²⁺) in an alkaline medium. These ferrous ions (Fe²⁺) react with thioglycolic acid, forming a pink or reddish-purple color complex.
The reaction is as follows:
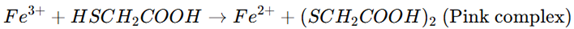
- – Thioglycolic acid acts as a reducing agent, converting Fe³⁺ to Fe²⁺.
- – Citric acid is used to prevent precipitation of iron salts, ensuring accurate color development.
- – Ammonia solution (alkaline medium) enhances the reaction, producing a stable pink to purple color.
- – The intensity of the color is visually compared with the standard iron solution.
Reagents Required
- 1. Standard Iron Solution (20 ppm Fe)
- 2. Thioglycolic Acid (0.1 ml)
- 3. Iron-Free Citric Acid Solution (20% w/v, 2 ml)
- 4. Iron-Free Ammonia Solution
- 5. Water
- 6. Test Substance (Sample Drug)
Procedure for the Limit Test for Iron
The procedure involves preparing a test solution and a standard iron solution in Nessler cylinders and comparing their color intensity.
Step-by-Step Procedure
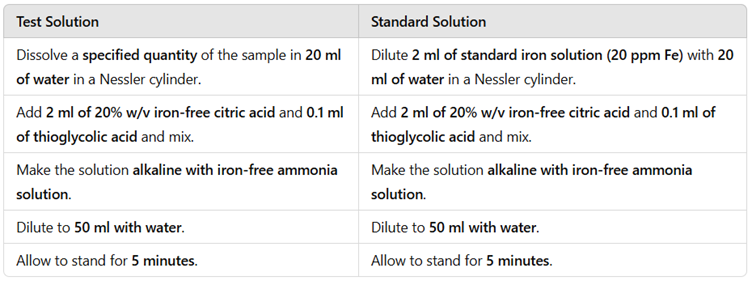
Observation
- (a) The color of the test solution is visually compared to the standard iron solution.
- (b) If the pink or purple color intensity of the test solution is equal to or lighter than the standard, the sample passes the test.
- (c) If the test solution is darker, it indicates excess iron impurities, and the sample fails the test.
Interpretation of Results
- Pass: The test solution’s pink or purple color is lighter than or equal to the standard, meaning the iron content is within the permissible limit.
- Fail: If the test solution is darker than the standard, it means the iron content exceeds the allowable limit, making the sample unsuitable for pharmaceutical use.
Significance of the Limit Test for Iron
1. Ensures Drug Purity
This test ensures that pharmaceutical substances do not contain excess iron, which can cause instability and degradation.
2. Compliance with Regulatory Standards
Pharmacopoeias such as IP, BP, and USP require the limit test for iron to maintain quality control in drug formulations.
3. Prevents Drug Discoloration
Iron contamination can cause undesirable color changes in pharmaceutical products, affecting their appearance and acceptance.
4. Protects Drug Stability
High iron levels can accelerate the oxidation of active pharmaceutical ingredients (APIs), leading to reduced potency and shorter shelf life.
5. Ensures Drug Safety
Iron contamination in excess can lead to toxicity, organ damage, and drug ineffectiveness. This test ensures that only safe levels of iron are present in the formulation.
6. Quality Control in Manufacturing
Pharmaceutical companies conduct this test to monitor raw materials, production processes, and final products for iron contamination.
Common Errors and Precautions
(1) Errors in the Test
- Impure Reagents – Ensure all solutions are iron-free to prevent false positives.
- Improper Alkaline Medium – The reaction requires an iron-free ammonia solution for accurate color development.
- Incorrect Dilution – Precise dilution is essential for reliable comparisons.
(2) Precautions to Follow
- Use freshly prepared reagents to prevent inaccurate results.
- Keep solutions free from contamination to avoid false positives.
- Conduct the test under consistent lighting conditions for accurate visual comparison.
Conclusion
The Limit Test for Iron is a critical pharmaceutical quality control test used to detect and limit iron impurities in drug substances. Based on the color reaction between iron and thioglycolic acid, this test ensures drugs meet regulatory standards for purity and stability. By implementing this test, pharmaceutical manufacturers can maintain high-quality formulations, enhance drug safety, and comply with global pharmacopeial guidelines.