Introduction
Metal ion indicators are chemical compounds that exhibit a distinct color change when they interact with metal ions. These indicators are widely used in complexometric titrations, especially in EDTA titrations, to determine the concentration of metal ions in a solution. The color change occurs due to the formation or dissociation of a metal-indicator complex.
Characteristics of an Ideal Metal Ion Indicator
A good metal ion indicator should:
- Exhibit a sharp and distinct color change at the endpoint of the titration.
- Form a stable but not too strong complex with the metal ion.
- Have a high selectivity for specific metal ions.
- Function effectively within a wide pH range.
- Be water-soluble and chemically stable in the titration medium.
Mechanism of Action
- The indicator initially binds to the metal ion, forming a metal-indicator complex, which has a specific color.
- As the titration progresses, a stronger chelating agent, such as EDTA (ethylenediaminetetraacetic acid), replaces the indicator, forming a more stable metal-EDTA complex.
- This causes the indicator to return to its free form, leading to a color change, which signals the endpoint of the titration.
Types of Metal Ion Indicators
Several metal ion indicators are used based on their specificity for different metal ions. The most commonly used indicators include:
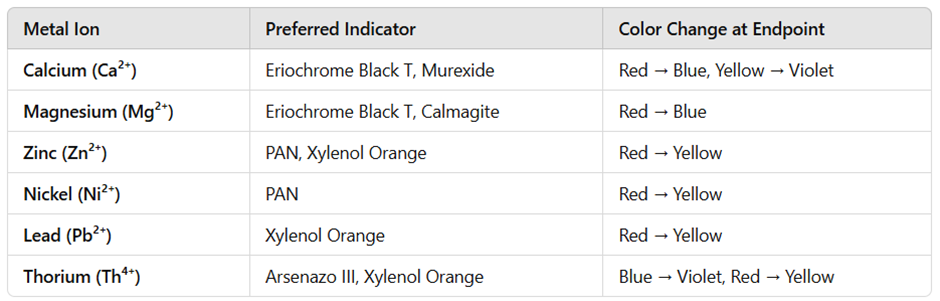
1. Eriochrome Black T (EBT)
- Used for titration of calcium (Ca²⁺), magnesium (Mg²⁺), and other alkaline earth metals.
- Appears blue in free form and forms a red complex with metal ions.
- In an EDTA titration, the endpoint is marked by a red-to-blue color change.
- Works best in the pH range of 7–11, often in an ammonia-ammonium chloride buffer.
2. Murexide (Ammonium Purpurate)
- Used primarily for calcium (Ca²⁺) and rare earth metal titrations.
- Changes from yellow to violet at the endpoint.
- Suitable for titrations in slightly acidic to neutral conditions.
3. Calmagite
- Alternative to Eriochrome Black T for calcium and magnesium titrations.
- Shows a red-to-blue color change at the endpoint.
- Useful in hard water analysis.
4. Xylenol Orange
- Highly sensitive to lanthanides and heavy metals such as lead (Pb²⁺) and thorium (Th⁴⁺).
- Changes from red to yellow at the endpoint.
- Works best in a pH range of 1.5–3.5.
5. PAN (1-(2-Pyridylazo)-2-naphthol)
- Used for detecting zinc (Zn²⁺), cobalt (Co²⁺), and nickel (Ni²⁺).
- Forms a red complex with metal ions and turns yellow at the endpoint.
- Effective in slightly acidic conditions.
6. Arsenazo III
- Used for titrations of actinides, thorium (Th⁴⁺), and uranium (UO₂²⁺).
- Color change from blue to violet at the endpoint.
- Works in highly acidic conditions (pH < 2).
Selection of Indicators for Specific Metal Ions
Factors Affecting Indicator Performance
- pH of the Solution: Some indicators require a specific pH range to function properly (e.g., Eriochrome Black T works best in alkaline conditions).
- Buffering System: A suitable buffer (e.g., ammonia buffer for Ca²⁺/Mg²⁺ titration) helps maintain the necessary pH.
- Selectivity: Some indicators may interact with multiple metal ions, requiring masking agents to prevent interference.
- Solubility: The indicator should be sufficiently soluble in the titration medium for effective color detection.
Applications of Metal Ion Indicators
- Water Hardness Analysis: Determination of Ca²⁺ and Mg²⁺ in water using EDTA titration with Eriochrome Black T.
- Pharmaceutical Analysis: Estimation of metal ion impurities in drug formulations.
- Industrial Use: Quality control in metal plating, mining, and chemical industries.
- Biological Studies: Detection of essential metal ions like Zn²⁺, Fe²⁺, and Ca²⁺ in biological fluids.
- Environmental Monitoring: Measuring toxic metal levels (Pb²⁺, Hg²⁺, Cd²⁺) in water and soil samples.
Conclusion
Metal ion indicators are indispensable tools in complexometric titrations, enabling accurate determination of metal ion concentrations through distinct color changes. The selection of an appropriate indicator depends on factors like pH, metal ion specificity, and complex stability. Their applications extend across analytical chemistry, pharmaceuticals, industrial processes, and environmental science, making them essential for various quantitative analyses.




