Naphthalene constitutes the largest part of coal-tar, accounting for approximately 6-10%. The hydrocarbon was initially noticed as a residue in the condensers during the distillation of the naphtha portion, hence its name. It is typically obtained by cooling the middle oil fraction (160-230°), where naphthalene crystallizes. The raw crystals are removed through centrifugation.
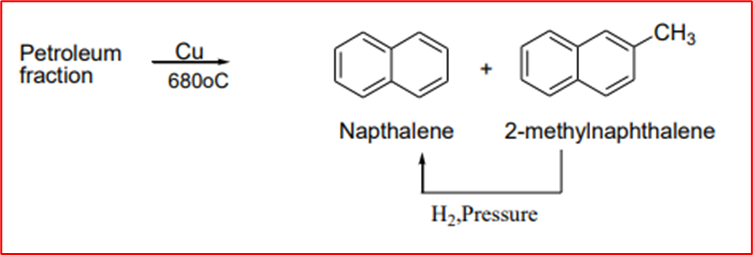
1. From petroleum: Naphthalene and methylnaphthalene are formed when petroleum fractions are passed over the copper catalyst at 680°C. The methylnaphthalene obtained is further converted into naphthalene by heating with hydrogen under reduced pressure (dealkylation reaction).
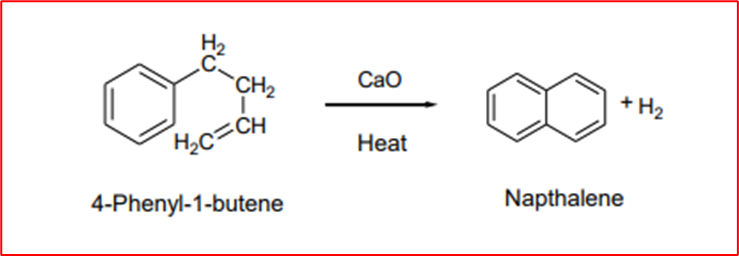
2. From 4-phenyl-1-butene: Naphthalene is obtained when 4-phenyl-1-butene is passed over red-hot calcium oxide.
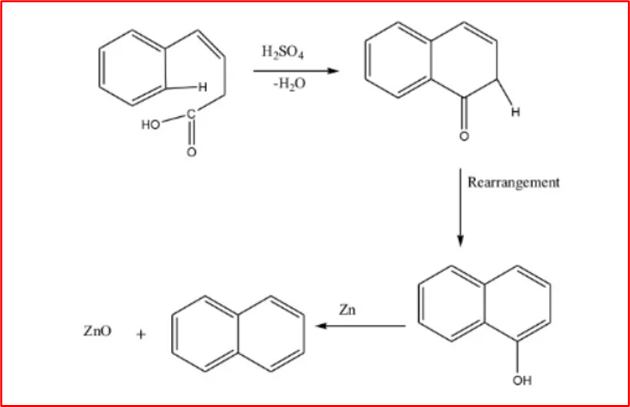
3. From 4-pheyl-3-butenoic acid: When 4-phenyl-3-butenoic acid is heated with concentrated sulfuric acid, 1-naphthol is formed. Distilling 1-naphthol with zinc dust results in the formation of naphthalene.
4. By Haworth synthesis: This reaction consists of 5 steps.
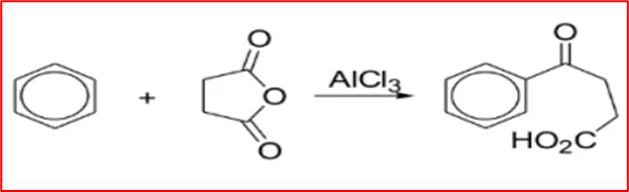
Step-I (Friedal Craft acylation): Benzene and succinic anhydride react with aluminium chloride to form β-benzoylpropionic acid.
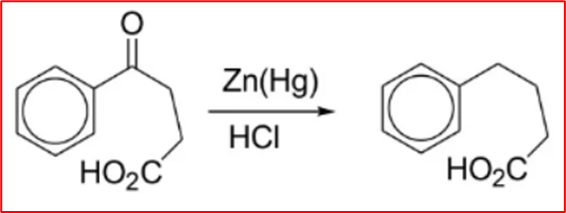
Step-II (Clemmenson reduction): β-Benzoylpropionic acid reacts with zinc amalgam in hydrochloric acid to yield 4-Phenylbutyric acid.
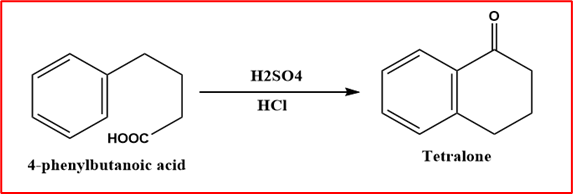
Step-III (Ring Closure reaction): When 4-Phenylbutyric acid is heated with concentrated Sulphuric acid or polyphosphoric acid, it forms α-tetralone.
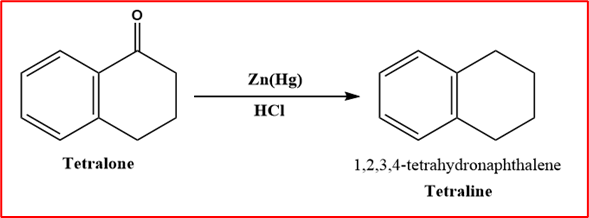
Step-IV (Clemmenson reduction): When Tetralone is heated with amalgamated zinc or HCl, a chemical reaction forms a tetraline compound.
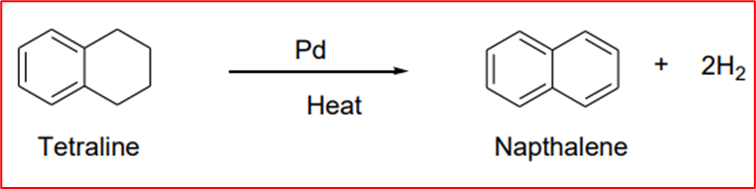
Step-V(Aromatization): When Tetraline is heated with palladium, it produces naphthalene.
Conclusion
Naphthalene, a significant component of coal tar, is prepared through various methods, including distillation from petroleum fractions, conversion of organic compounds like 4-phenyl-1-butene and 4-phenyl-3-butenoic acid, and the Haworth synthesis. The latter involves a series of reactions, including Friedel-Crafts acylation, Clemmenson reduction, ring closure, and aromatization. Each method offers distinct advantages and may be chosen based on factors such as the availability of starting materials and the desired purity of the final product.




