Introduction
Concentration is a measure of the amount of a substance (solute) present in a given quantity of solution or solvent. It is a crucial parameter in pharmaceutical analysis, as accurate concentration measurements ensure proper drug formulation, quality control, and regulatory compliance. Various methods are used to express concentration depending on the type of solution and the precision required.
1. Mass-Based Expressions
These methods express the concentration in terms of the mass (weight) of the solute and solvent or solution.
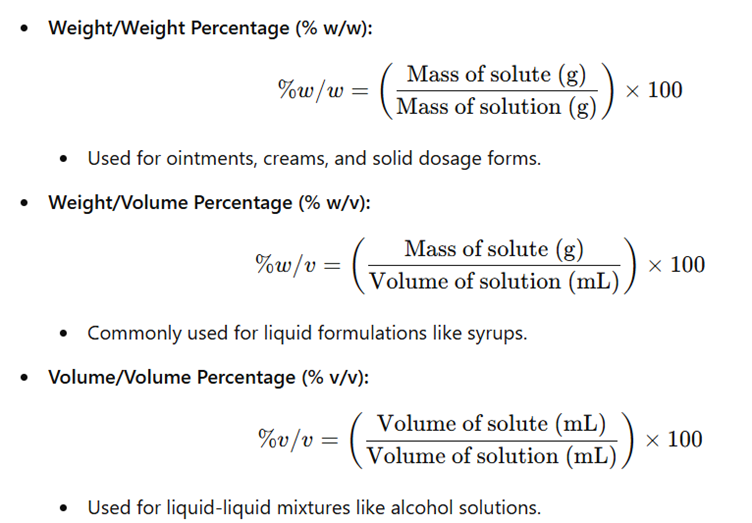
a) Percentage Concentration (% w/w, % w/v, % v/v)
2. Molar Concentration-Based Expressions
These methods express concentration in terms of the number of moles of solute present.
a) Molarity (M)
- Defined as the number of moles of solute present in one litter of solution.
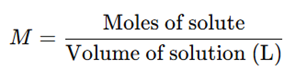
- Commonly used in analytical chemistry and pharmaceutical formulations.
b) Molality (m)
- Defined as the number of moles of solute per kilogram of solvent.

- Used in cases where temperature variation affects volume but not mass (e.g., cryoscopic and boiling point elevation studies).
c) Normality (N)
- Expresses concentration in terms of gram equivalents of solute per liter of solution.

- Used in acid-base titrations, redox reactions, and precipitation reactions.
d) Formality (F)
- Similar to molarity but used for substances that do not form true molecular solutions (e.g., ionic compounds).
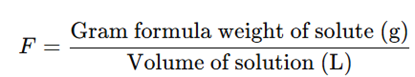
3. Mole Fraction (X)
- Defined as the ratio of moles of one component to the total number of moles in the solution.
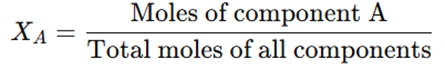
- Used in thermodynamic calculations and vapor pressure studies.
4. Parts Per Expressions
These methods are used for expressing very small concentrations, such as in environmental and pharmaceutical contamination analysis.
a) Parts Per Million (ppm)
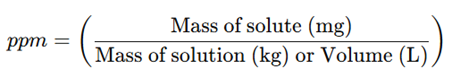
- 1 ppm = 1 mg of solute per 1 L of solution or 1 kg of solvent.
- Used for trace impurity analysis in drugs and water quality testing.
b) Parts Per Billion (ppb)

- 1 ppb = 1 μg of solute per 1 L of solution or 1 kg of solvent.
- Used in ultra-trace analysis of contaminants.
5. Osmolarity and Osmolality
These methods measure the number of osmotically active particles in a solution, important in biological and pharmaceutical formulations.
a) Osmolarity (Osm/L)
- Number of osmoles of solute per liter of solution.
b) Osmolality (Osm/kg)
- Number of osmoles of solute per kilogram of solvent.
- Used in intravenous fluid and electrolyte balance studies.
Conclusion
Different methods are used to express concentration based on the requirements of pharmaceutical analysis. Mass-based methods are used in formulations, molar-based methods in chemical reactions, and trace-level methods in impurity analysis. Understanding these expressions ensures accurate drug formulation, dosing, and quality control.




Well explained.
Dude 😎 good working for helping students.
Thanks and keep updating.