Introduction
Potentiometric titration is an analytical method that determines the concentration of an analyte by measuring the electrode potential during titration. Unlike color-based indicators in conventional titrations, potentiometric titrations use electrodes to detect the endpoint without requiring visual indicators.
Methods to Determine the End Point of Potentiometric Titration
The endpoint of a potentiometric titration is detected by analyzing how the electrode potential (E) changes with the addition of titrant. Several mathematical and graphical techniques help identify the endpoint accurately.
1. First Derivative Method
Principle:
- The first derivative (ΔE/ΔV) represents the rate of change of electrode potential with respect to the volume of titrant added (V).
- The maximum peak in the first derivative curve corresponds to the endpoint.
Procedure:
- Measure the potential after each addition of titrant.
- Plot the first derivative curve:
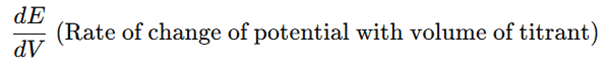
- The peak of the curve corresponds to the endpoint.
Advantages:
✔ More accurate than direct potential measurement.
✔ Useful for titrations with a gradual potential change near the endpoint.
2. Second Derivative Method
Principle:
- The second derivative (d2E/dV2) identifies the point of greatest curvature change in the titration curve.
- At the endpoint, the second derivative crosses zero, which makes it easier to pinpoint the exact endpoint.
Procedure:
- Measure potential after each addition of titrant.
- Calculate the second derivative:
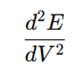
- The zero-crossing point in the second derivative curve indicates the endpoint.
Advantages:
✔ More precise than the first derivative method, especially in weak titrations.
✔ Useful for titrations with slow potential change near the endpoint.
3. Gran’s Plot Method
Principle:
- Gran’s plot is a graphical method that extrapolates the linear portion of the titration curve to determine the endpoint.
- It is mainly used for acid-base titrations and redox titrations.
Procedure:
- Plot a graph of (V × 10E) vs. volume of titrant added.
- The extrapolated intersection of the linear portion determines the endpoint.
Advantages:
✔ High precision for weak acid-base and redox titrations.
✔ Useful when other methods fail to give a clear endpoint.
4. Inflection Point Method (Graphical Method)
Principle:
- The inflection point in a potentiometric titration curve is the sharpest potential change.
- The steepest part of the titration curve corresponds to the endpoint.
Procedure:
- Plot E vs. volume of titrant (V).
- The endpoint is determined at the sharpest potential jump.
Advantages:
✔ Simple and effective for strong acid-strong base titrations.
✔ Provides graphical confirmation of the endpoint.
Applications of Potentiometric Titration
Potentiometric titration is widely used in pharmaceutical, environmental, industrial, and biological analysis due to its high accuracy and reliability.
1. Acid-Base Titrations
- Used for strong acid-strong base and weak acid-strong base titrations.
- Example: Determination of HCl using NaOH.
2. Redox Titrations
- Used for oxidation-reduction (redox) reactions.
- Example: Determination of Fe²⁺ using KMnO₄ in pharmaceutical analysis.
3. Precipitation Titrations
- Used to determine the concentration of halide ions (Cl⁻, Br⁻, I⁻) by precipitation with AgNO₃.
- Example: determination of chloride in water samples.
4. Complexometric Titrations
- Used to determine metal ions using EDTA titration.
- Example: Determination of Ca²⁺ and Mg²⁺ in water hardness analysis.
5. Pharmaceutical Analysis
- Used for purity testing and assay determination of drug substances.
- Example: Potentiometric titration of aspirin for its purity.
6. Environmental Analysis
- Used in water quality testing, determination of pollutants, and toxic metal detection.
- Example: Determination of fluoride in drinking water.
Conclusion
Potentiometric titration is a precise and versatile analytical technique that determines titration endpoints without visual indicators.
- Different endpoint detection methods (First & Second Derivative, Gran’s Plot, Inflection Point) improve accuracy.
- It is widely used in acid-base, redox, precipitation, and complexometric titrations across pharmaceutical, environmental, and industrial applications.
This method ensures high accuracy, minimal sample interference, and suitability for colored or turbid solutions, making it superior to traditional titrations.