Introduction
The Modified Volhard’s Method is an adaptation of the classical Volhard’s Method, used for the determination of chloride (Cl⁻), bromide (Br⁻), and iodide (I⁻) ions. This modification eliminates the need for filtration by using a protective colloid (e.g., nitrobenzene, dextrin, or ferric salts) to prevent the re-dissolution of precipitated silver halides (AgCl, AgBr, AgI). This method is particularly useful when dealing with turbid solutions or samples containing interfering substances that could dissolve the silver halide precipitate.
Principle of Modified Volhard’s Method
The modified Volhard’s method follows the same basic steps as the traditional Volhard’s method but prevents the redissolution of silver halide precipitate by adding a stabilizing agent (protective colloid). This modification ensures a more accurate endpoint detection without the need for filtration.
Key Modifications in the Method
- Addition of Nitrobenzene or Dextrin: These act as protective colloids, preventing the redissolution of silver halides and ensuring accurate results.
- Precipitation in a Homogeneous Solution: This improves endpoint clarity and prevents errors due to re-dissolved AgCl, AgBr, or AgI.
- Better Control Over Endpoint: The red-colored ferric thiocyanate complex remains stable, leading to sharper endpoint detection.
Chemical Reactions in Modified Volhard’s Method
Step 1: Precipitation of Chloride/Bromide/Iodide
- The sample containing Cl⁻, Br⁻, or I⁻ is treated with an excess known volume of AgNO₃ in the presence of a protective colloid.

The protective colloid prevents AgCl, AgBr, or AgI from redissolving.
Step 2: Back Titration with KSCN (Potassium Thiocyanate)
- The excess AgNO₃ remaining in the solution is titrated with KSCN.
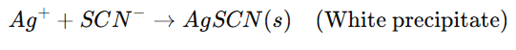
Step 3: Endpoint Detection Using Ferric Indicator
- Once the reaction consumes all Ag⁺, the next drop of KSCN reacts with ferric ammonium sulfate (Fe³⁺) to form a red ferric thiocyanate complex, marking the endpoint.
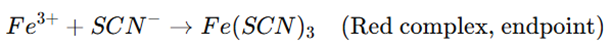
Procedure of Modified Volhard’s Method
1. Preparation of Solutions
- Standard Silver Nitrate Solution (AgNO₃, 0.1 N): Used as a titrant for halide ions.
- Standard Potassium Thiocyanate Solution (KSCN, 0.1 N): Used for back-titration.
- Ferric Ammonium Sulfate (Indicator, 5% solution): Used for endpoint detection.
- Protective Colloid (Nitrobenzene/Dextrin): Prevents re-dissolution of silver halides.
2. Titration Procedure
- Pipette a known volume of the sample solution into a conical flask.
- Add excess AgNO₃ solution to ensure complete precipitation of halides.
- Add the protective colloid (e.g., nitrobenzene or dextrin) to stabilize the silver halide precipitate.
- Titrate the excess AgNO₃ with KSCN, using ferric ammonium sulfate as an indicator.
- Endpoint Detection: The first appearance of a persistent red color due to Fe(SCN)₃ complex indicates the endpoint.
Calculation
Calculate the concentration of chloride, bromide, or iodide using:

The mass of halide ion can be calculated as:

Advantages of Modified Volhard’s Method
- No Need for Filtration: Protective colloids prevent the re-dissolution of silver halides.
- Improved Accuracy: Less chance of endpoint errors caused by re-dissolved AgCl, AgBr, or AgI.
- More Stable Endpoint: The red Fe(SCN)₃ complex appears more sharply.
- Applicable to a Wide Range of Halide Concentrations.
- Can Be Used in Turbid or Complex Samples.
Limitations of Modified Volhard’s Method
- Requires Additional Stabilizing Agents: Nitrobenzene or dextrin must be carefully added.
- Longer Reaction Time: Protective colloids slow down precipitation slightly.
- Not Suitable for Highly Basic Solutions: Ferric hydroxide (Fe(OH)3) may precipitate.
- Careful Control of Colloid Amount Needed: Too much colloid can interfere with precipitation.
Applications of Modified Volhard’s Method
- Water Analysis: Used for determining chloride content in water and wastewater.
- Pharmaceutical Analysis: Quantification of halides in drugs and saline solutions.
- Food and Beverage Industry: Determining salt content in food products.
- Environmental Testing: Chloride, bromide, and iodide estimation in soil and industrial effluents.
- Biological Fluids: Analysis of chloride content in blood serum or urine samples.
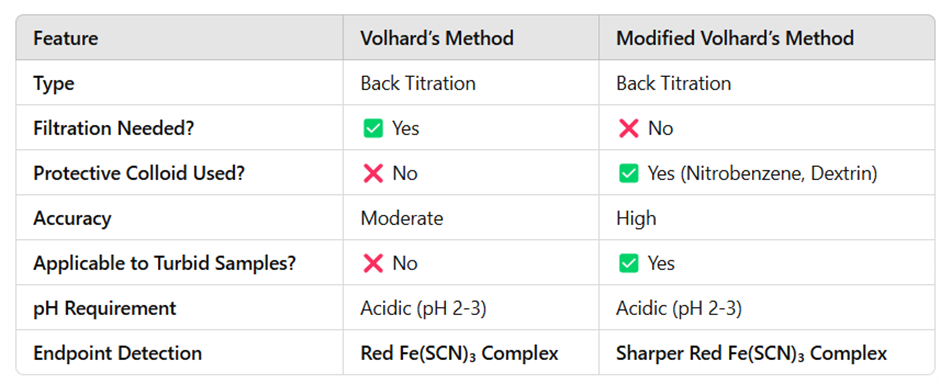
Comparison: Volhard’s vs. Modified Volhard’s Method
Conclusion
The Modified Volhard’s Method is a significant improvement over the traditional Volhard’s Method, as it eliminates the need for filtration by stabilizing silver halide precipitates using protective colloids like nitrobenzene or dextrin. This makes it highly effective for turbid solutions and complex samples while maintaining accuracy in halide determination.