Introduction
Mohr’s method is a classical argentometric precipitation titration used for the quantitative determination of chloride (Cl⁻) and bromide (Br⁻) ions using silver nitrate (AgNO₃) as a titrant. Potassium chromate (K₂CrO₄) acts as an indicator, which helps detect the endpoint by forming a reddish-brown precipitate of silver chromate (Ag₂CrO₄).
Principle of Mohr’s Method
Mohr’s method is based on the formation of an insoluble silver chloride (AgCl) or silver bromide (AgBr) precipitate when silver nitrate (AgNO₃) is added to a solution containing chloride (Cl⁻) or bromide (Br⁻). The titration continues until all the chloride or bromide ions react with the silver ions. Once the chloride or bromide is completely precipitated, the first excess drop of AgNO₃ reacts with the chromate indicator to form a reddish-brown precipitate of silver chromate (Ag₂CrO₄), which indicates the endpoint.
Chemical Reactions in Mohr’s Method
- Precipitation Reaction (During Titration):

- Endpoint Detection (After Complete Precipitation of Cl⁻ or Br⁻):

The appearance of this reddish-brown precipitate of Ag₂CrO₄ marks the endpoint of the titration.
Procedure of Mohr’s Method
1. Preparation of Solutions
- Standard Silver Nitrate Solution (AgNO₃, 0.1 N): Dissolve accurately weighed AgNO₃ in distilled water and store in an amber-colored bottle (light-sensitive).
- Potassium Chromate Indicator (K₂CrO₄, 5% solution): Prepare a fresh solution and filter to remove any impurities.
- Sample Solution: The unknown chloride/bromide solution is prepared and acidified with a small amount of dilute nitric acid (HNO₃) to prevent precipitation of other salts.
2. Titration Procedure
- Pipette a known volume of the sample solution into a conical flask.
- Add 1-2 mL of potassium chromate indicator (K₂CrO₄).
- Titrate the solution with AgNO₃ from a burette while stirring continuously.
- Endpoint Detection: Stop adding AgNO₃ once a persistent reddish-brown color appears.
- Record the volume of AgNO₃ used.
Calculation
The concentration of chloride (Cl⁻) or bromide (Br⁻) in the sample solution is calculated using the formula:

If molarity (M) is used instead of normality:
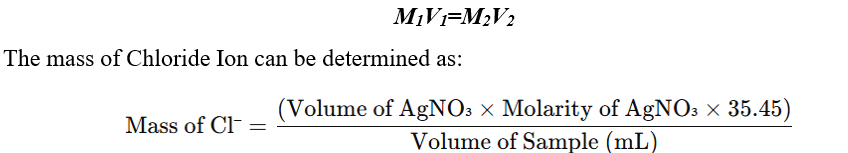
Factors Affecting Mohr’s Method
1. pH of the Solution
- The titration should be conducted at a pH between 6.5 and 10.
- Too acidic (pH < 6.5): H⁺ ions react with chromate (CrO42−) to form chromic acid (H2CrO4), reducing the availability of chromate for endpoint detection.
- Too basic (pH > 10): Silver ions (Ag+) may react with hydroxide ions (OH⁻) to form silver hydroxide (AgOH), leading to interference.
2. Presence of Other Ions
- Ions like phosphate (PO₄³⁻), sulfate (SO₄²⁻), or carbonate (CO₃²⁻) may form precipitates with silver, leading to inaccurate results.
- Other halides (e.g., iodide, bromide) can interfere.
3. Solubility of Silver Chromate (Ag₂CrO₄)
- The endpoint should be detected immediately because silver chromate (Ag2CrO4Ag₂CrO₄) is slightly soluble in water and may dissolve back, causing endpoint errors.
Advantages of Mohr’s Method
- Simple and Direct: No need for back-titration or additional complexing agents.
- Reliable for Cl⁻ and Br⁻ Determination: Provides accurate results for chloride and bromide ions.
- Easy Endpoint Detection: The reddish-brown precipitate is visually distinct.
Limitations of Mohr’s Method
- Cannot Be Used for Iodide (I⁻) or Cyanide (CN⁻): Silver iodide (AgI) and silver cyanide (AgCN) are too insoluble, making endpoint detection difficult.
- Sensitive to pH Changes: Requires careful pH control to prevent interference.
- Interference from Other Anions: Sulfates, phosphates, or carbonates can precipitate with silver ions, affecting accuracy.
Applications of Mohr’s Method
- Water analysis: determination of chloride content in drinking water and wastewater.
- Pharmaceutical Analysis: Quantification of chloride in drugs and saline solutions.
- Food and Beverage Industry: Estimation of salt content in food products.
- Environmental Testing: Monitoring chloride levels in soil and industrial effluents.
Conclusion
Mohr’s method is a widely used and reliable precipitation titration for the determination of chloride and bromide ions. It relies on the precipitation of silver halides and the use of potassium chromate as an indicator. While simple and effective, it requires careful control of pH and interference from other ions for accurate results.