Naphthalene is a white, crystalline solid with a distinct odor reminiscent of mothballs. It slowly sublimes at room temperature, generating a highly flammable vapor. John Kidd (1775–1851), an English chemist and physician, is credited with discovering naphthalene from coal tar in 1819. It contains a complex hydrocarbon mixture similar to what is found in petroleum. Richard August Carl Emil Erlenmeyer, a German chemist, discovered the chemical structure of naphthalene. According to Erlenmeyer, the naphthalene molecule is composed of two benzene molecules linked together.
Structure of Naphthalene
Naphthalene is a type of polynuclear aromatic hydrocarbon in which two aromatic benzene rings are fused at the ortho position.
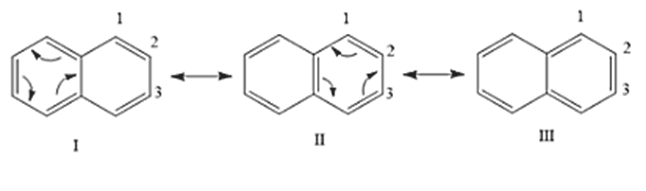
The bond length at the double bond position is approximately 1.36 Angstrom. The bond length at the single bond position is approximately 1.40 Angstrom. The structure of the naphthalene molecule exhibits resonance, forming a resonance hybrid. There are three resonance structures for naphthalene molecules.
Naphthalene possesses distinct physical properties as outlined below:
- Naphthalene typically appears in a crystalline state.
- It exhibits a predominantly white color, although it can also be found in variations ranging from transparent to brownish hues.
- Naphthalene is relatively light, With a molecular weight of 128.18 g/mol.
- At standard room temperature, naphthalene demonstrates low solubility in water.
- It is characterized by an aromatic odor, often described as distinctive.
- The vapor pressure of naphthalene measures approximately 0.087 mmHg.
Conclusion
Naphthalene is a white, crystalline compound with a distinct mothball-like odor. Discovered by John Kidd in 1819, its chemical structure was elucidated by Richard August Carl Emil Erlenmeyer. Composed of fused benzene rings, naphthalene exhibits resonance and possesses unique physical properties, including low solubility in water and a relatively light molecular weight. These characteristics contribute to its diverse industrial and household applications.




