Theory of orientation:
Orientation or directive effect can be explained by studying all the possible resonance structures of the sigma complex formed due to the electrophile at ortho, meta, and para positions for different types of monosubstituted benzene.
a) Ortho-para directing groups which have an electron-releasing inductive effect (+I effect):
For example, alkyl groups (-R) have a +I effect, i.e., an electron-releasing result. Let’s study the sigma complexes (carbocation intermediate), which are formed by attack of electrophile (+E) at ortho, para, and meta position of mono-substituted benzene (toluene).
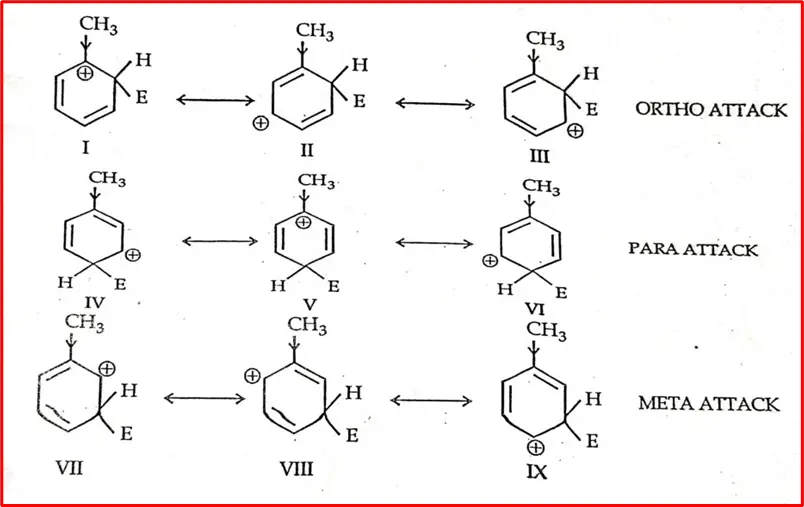
The sigma complex or carbocation intermediate is a resonance hybrid of three Structures. The Alkyl group has an electron-releasing effect, dispersing the positive charges and stabilizing the carbocation. Structure 1&5 effect is maximum because +ve order is present at the position of carbon where the methyl group is attached.
b) Ortho-para directing groups that have electron withdrawing (-I effect) and electron releasing (+R or +M effect) resonance or mesomeric effect:
Ex -NH2 group. Various sigma complexes or intermediates result from the attraction of electrophile Ortho-para and meta positions.
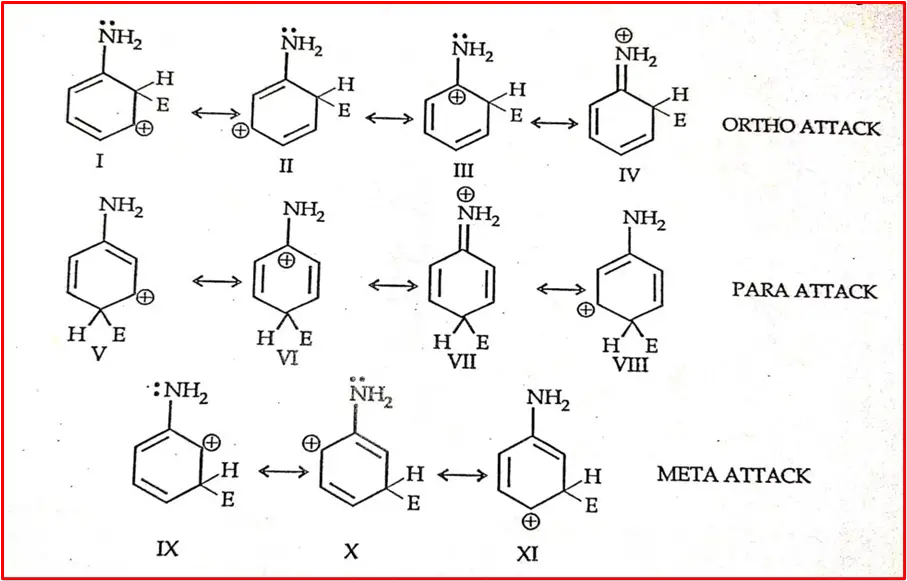
At Ortho and para positions, the sigma complex obtained a resonance hybrid of four Structures, at meta position only three. Structures 4 and 7 are more stable as positive charges delocalize on the nitrogen and ring carbon atoms.
c) Meta-directing groups:
All meta-directing groups (-NO2, CN, COOH, -CHO, -SO3H) are naturally electron-withdrawing. They have both electron-withdrawing inductive and resonance effects, i.e., the -I and -R effect.
Intermediate carbocation resulting from ortho, Para, and meta effect:
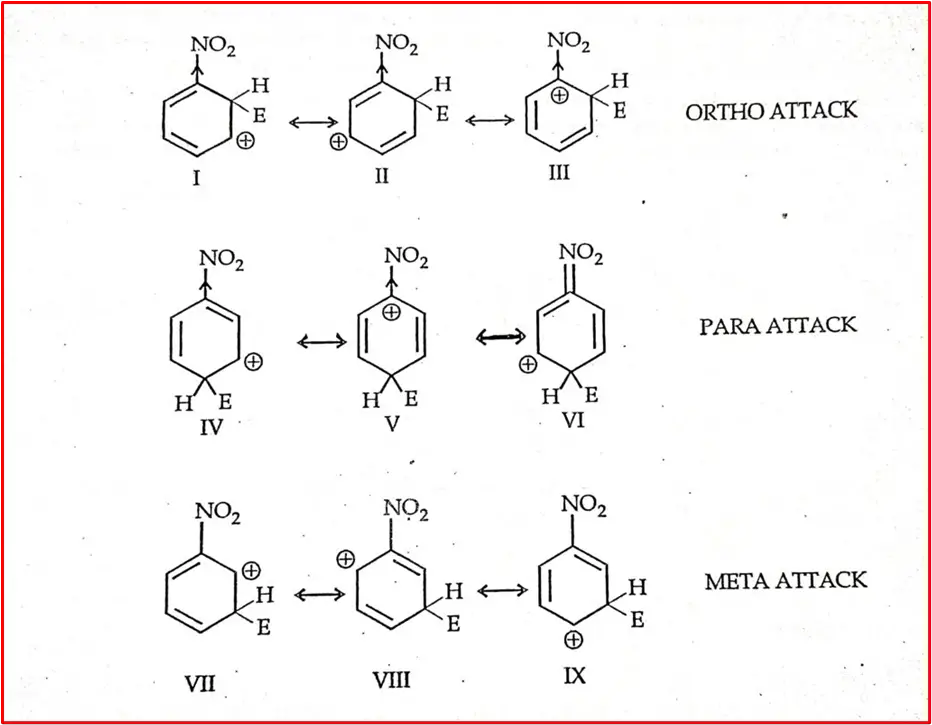
Structures 3 and 5 are highly unstable as electron-withdrawing nitro groups attached to the carbon atom have a +ve charge. So, the sigma complex intermediate from Ortho ¶ attacks a resonance hybrid of 2 Structure. Ortho-para attacking sigma complexes are less stable than meta-attacks. Electrophilic substitution reaction occurs more slowly in nitrobenzene than in Benzene.




