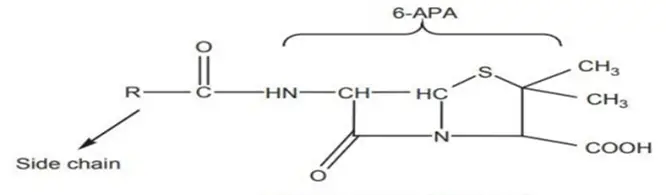
Penicillin, the most important antibiotic, was first extracted from the mould Penicillium notatum. Subsequently, a mutant of a related mould, P. chrysogenum, was found to give the highest yield of penicillin and is employed for the commercial production of this antibiotic. Penicillin belongs to a group of antibiotics called β-lactam antibiotics. The basic structure of the penicillins consists of a thiazolidine ring fused with a βlactam ring, which is essential for antibacterial activity. These two rings constitute the fundamental nucleus of all the penicillins, namely, 6-amino penicillanic acid (6-APA). Various semisynthetic penicillins are produced by altering the composition of the side chain attached to the 6-APA nucleus. Both the 6-APA nucleus and side chain are essential for antibacterial activity.
Historical Background of Penicillin
– Penicillin, the first antibiotic discovered, was serendipitously found by Scottish bacteriologist Alexander Fleming in 1928.
– Fleming noticed that a mold of the Penicillium genus produced a substance that inhibited the growth of Staphylococcus bacteria in a petri dish.
– However, it wasn’t until the 1940s that penicillin was successfully isolated, purified, and mass-produced by Howard Florey, Ernst Chain, and Norman Heatley.
– Penicillin’s introduction revolutionized medicine and drastically reduced mortality rates from bacterial infections.
Nomenclature of penicillin
(a) There are two types of numbering for the fused bicycling system of penicillin: whether which atom is number one Sulfur or Nitrogen.
(b) Penam nucleus is used in naming, which comprises a bicyclic system with the amide carbonyl group. Penicillin is named as 6-acylamino-2,2-dimethylpenam-3-carboxylic acid.
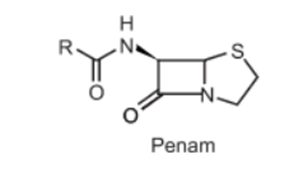
(c) Penicillanic acid nucleus: Which includes the 2,2-dimethyl and 3-carboxyl groups. Penicillin is named as 6- carbonylaminopenicillanic acid.
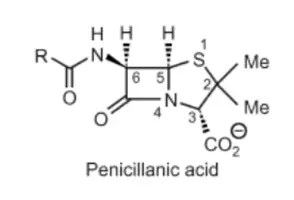
(d) Penicillin nucleus: Which includes 6-carbonyl aminopenicillanic acid. So Penicillin G is named benzylpenicillin if R is benzene ring.
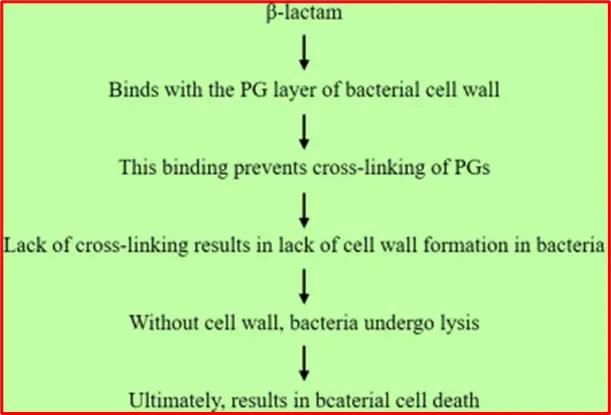
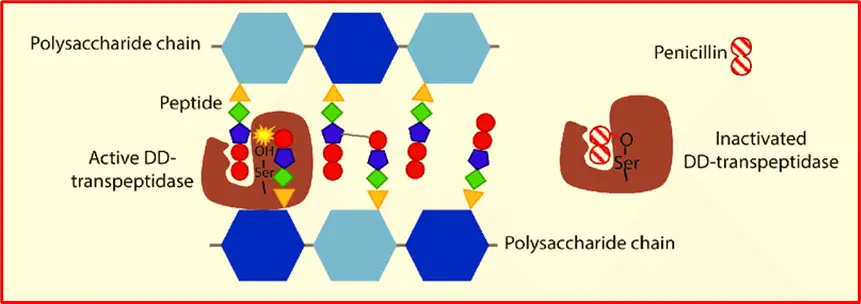
Mechanism of action of Penicillin
Penicillin and other beta-lactam antibiotics contain a four-membered beta-lactam ring. They work by irreversibly binding to DD-transpeptidase, an enzyme involved in bacterial cell wall synthesis. This binding inhibits the enzyme’s cross-linking activity, disrupting cell wall formation and leading to bacterial cell lysis and death.