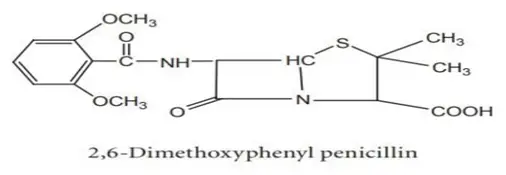
Penicillinase-resistant penicillins, also known as anti-staphylococcal penicillins, are a subclass of penicillin antibiotics designed to resist the action of beta-lactamase enzymes produced by some bacteria, particularly Staphylococcus aureus. These enzymes can break down the beta-lactam ring, rendering traditional penicillins ineffective. Here are the main penicillinase-resistant penicillins:
1. Methicillin:
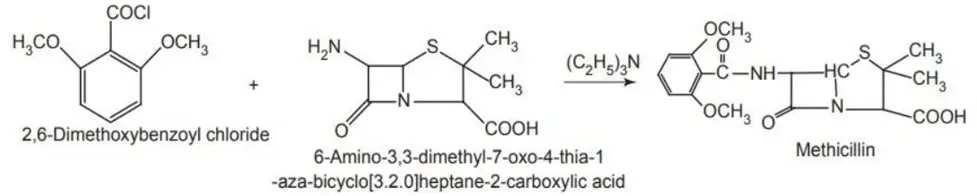
Synthesis of methicillin:
Properties and uses of methicillin
Methicillin sodium is a white crystalline solid with no odor. It is soluble in water and slightly soluble in chloroform, but insoluble in ether. Notably, it is highly resistant to inactivation by the penicillinase enzyme produced by Staphylococci bacteria and is even more resistant than penicillin G to penicillinase from Bacillus cereus.
Introduced for treating Staphylococci infections resistant to other penicillins, methicillin sodium is administered by intramuscular injection (IM) or by slow intravenous (IV) infusion every 4–6 hours.
2. Oxacillin (Isoxazolyl penicillins):
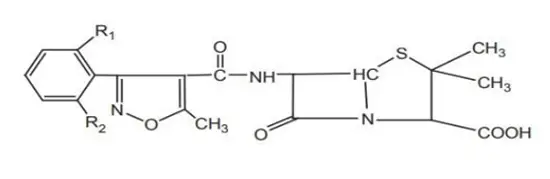
Synthesis of oxacillin:
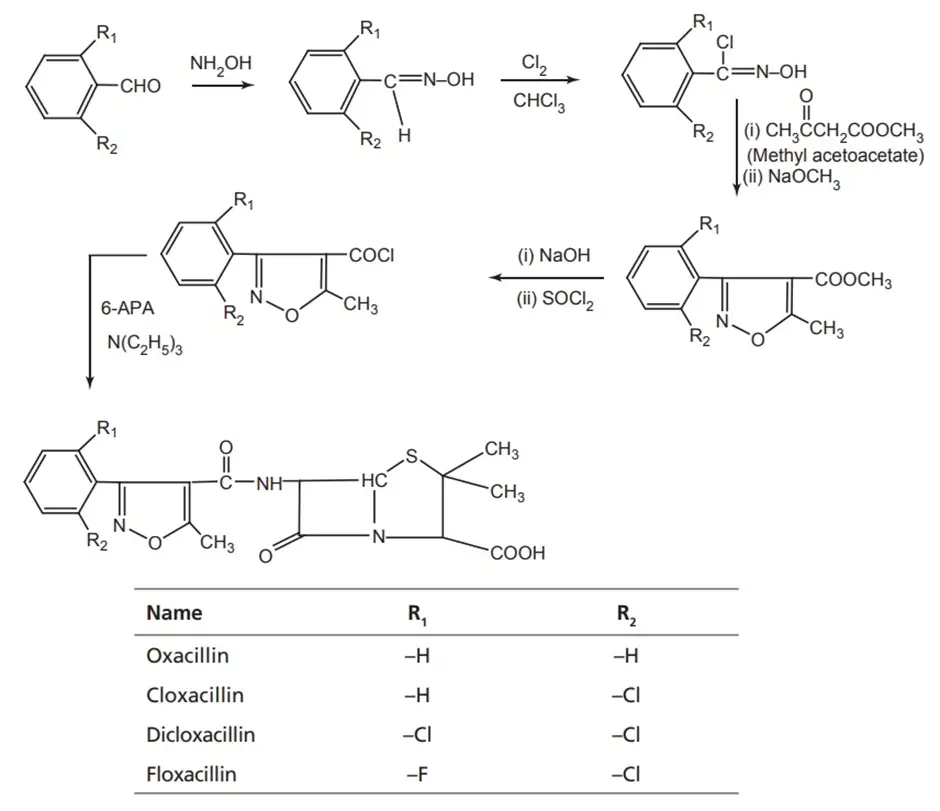
Properties and uses of Oxacillin
Penicillin V (Oxacillin) is a white, odorless, crystalline powder with a slightly bitter taste, soluble in water. It is more resistant to inactivation by gastric juice than penicillin G and is better absorbed from the gastrointestinal (GI) tract. Equivalent oral doses result in two to five times greater plasma concentration than penicillin G.
Penicillin V is administered to treat conditions such as ‘trench mouth’. It is also useful in treating streptococcal pharyngitis, pneumonia, arthritis, meningitis, and endocarditis caused by Streptococcus pyogenes.